Abstract
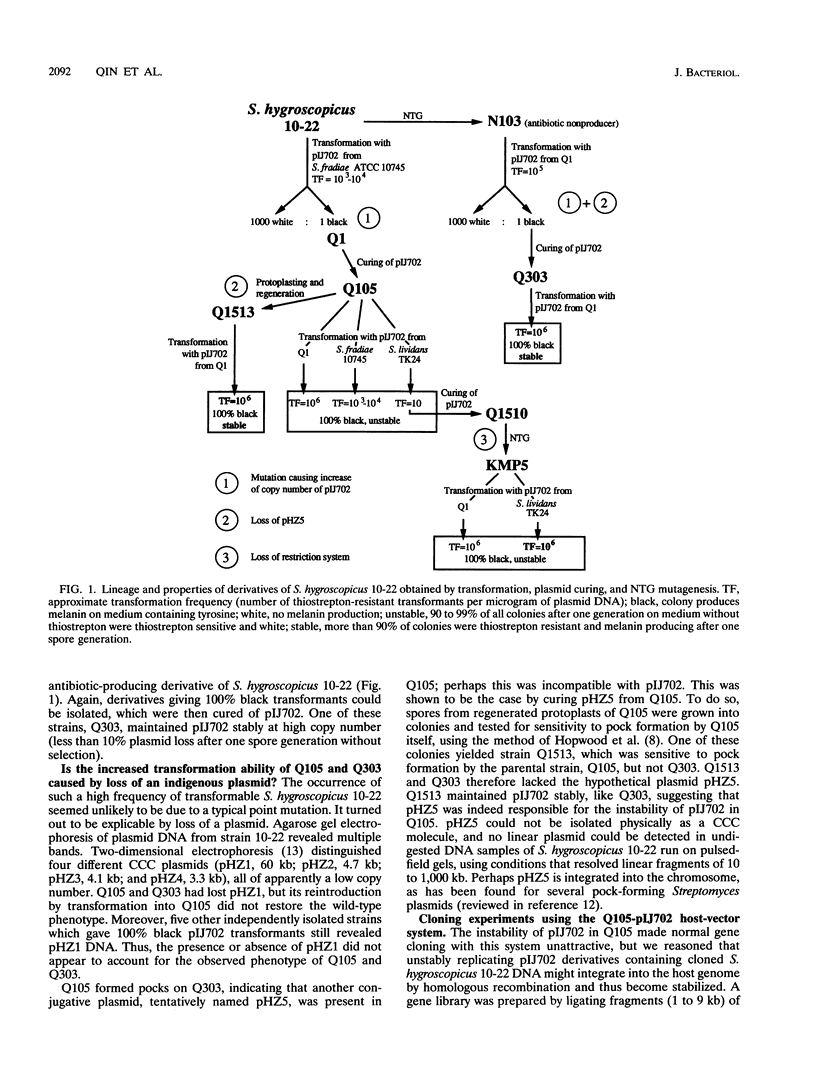
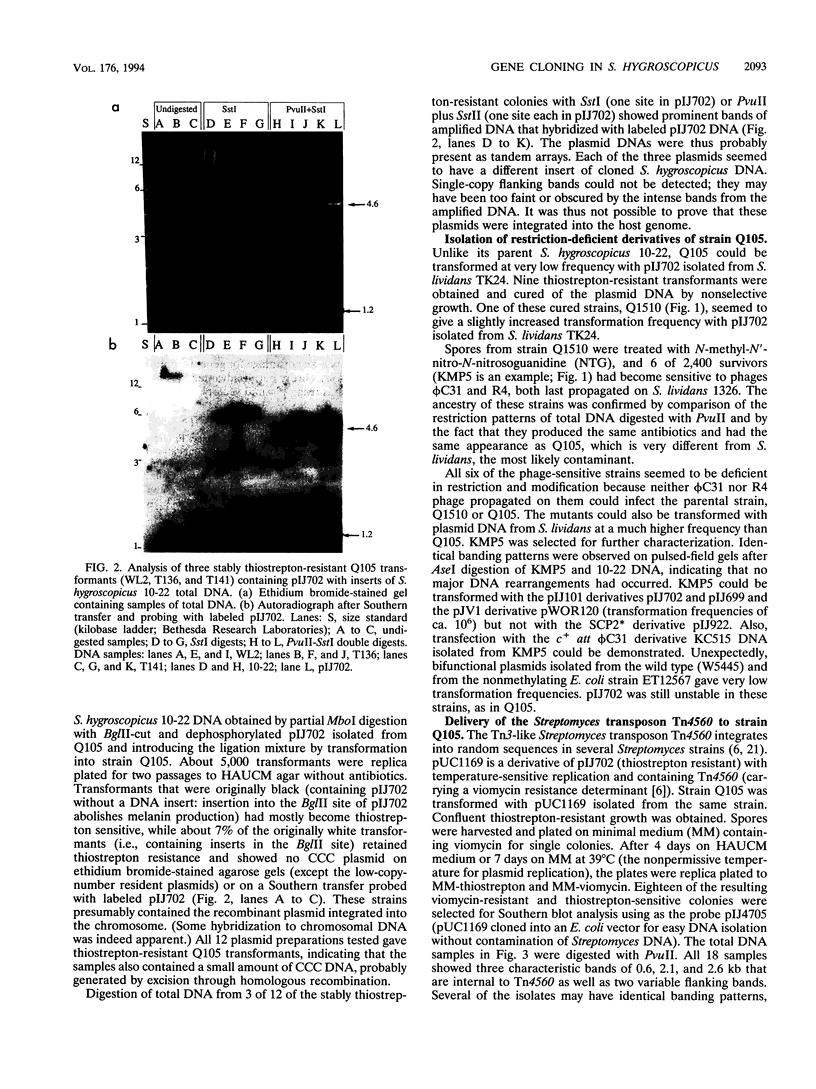
Streptomyces hygroscopicus 10-22 could not be transformed with any of the commonly used Streptomyces plasmid vectors and was resistant to plaque formation by the Streptomyces phages phi C31 and R4. Repeated selection resulted in the isolation of derivatives of S. hygroscopicus 10-22 that could be transformed with pIJ101- and pJV1-derived cloning vectors and of restriction-deficient derivatives that could accept DNA propagated in Streptomyces lividans 66. These new strains, which include three that still produce the original antibiotics, can be used as hosts for gene cloning. Insertion of nonreplicating vectors by homologous recombination and transposition of Tn4560 were demonstrated in S. hygroscopicus 10-22.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailey C. R., Bruton C. J., Butler M. J., Chater K. F., Harris J. E., Hopwood D. A. Properties of in vitro recombinant derivatives of pJV1, a multi-copy plasmid from Streptomyces phaeochromogenes. J Gen Microbiol. 1986 Aug;132(8):2071–2078. doi: 10.1099/00221287-132-8-2071. [DOI] [PubMed] [Google Scholar]
- Chater K. F., Bruton C. J. Mutational cloning in Streptomyces and the isolation of antibiotic production genes. Gene. 1983 Dec;26(1):67–78. doi: 10.1016/0378-1119(83)90037-9. [DOI] [PubMed] [Google Scholar]
- Chi N. Y., Ehrlich S. D., Lederberg J. Functional expression of two Bacillus subtilis chromosomal genes in Escherichia coli. J Bacteriol. 1978 Feb;133(2):816–821. doi: 10.1128/jb.133.2.816-821.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung S. T. Tn4556, a 6.8-kilobase-pair transposable element of Streptomyces fradiae. J Bacteriol. 1987 Oct;169(10):4436–4441. doi: 10.1128/jb.169.10.4436-4441.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopwood D. A., Kieser T., Wright H. M., Bibb M. J. Plasmids, recombination and chromosome mapping in Streptomyces lividans 66. J Gen Microbiol. 1983 Jul;129(7):2257–2269. doi: 10.1099/00221287-129-7-2257. [DOI] [PubMed] [Google Scholar]
- Katz E., Thompson C. J., Hopwood D. A. Cloning and expression of the tyrosinase gene from Streptomyces antibioticus in Streptomyces lividans. J Gen Microbiol. 1983 Sep;129(9):2703–2714. doi: 10.1099/00221287-129-9-2703. [DOI] [PubMed] [Google Scholar]
- Kieser H. M., Kieser T., Hopwood D. A. A combined genetic and physical map of the Streptomyces coelicolor A3(2) chromosome. J Bacteriol. 1992 Sep;174(17):5496–5507. doi: 10.1128/jb.174.17.5496-5507.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieser T. Factors affecting the isolation of CCC DNA from Streptomyces lividans and Escherichia coli. Plasmid. 1984 Jul;12(1):19–36. doi: 10.1016/0147-619x(84)90063-5. [DOI] [PubMed] [Google Scholar]
- Kieser T., Hopwood D. A. Genetic manipulation of Streptomyces: integrating vectors and gene replacement. Methods Enzymol. 1991;204:430–458. doi: 10.1016/0076-6879(91)04023-h. [DOI] [PubMed] [Google Scholar]
- Kieser T., Hopwood D. A., Wright H. M., Thompson C. J. pIJ101, a multi-copy broad host-range Streptomyces plasmid: functional analysis and development of DNA cloning vectors. Mol Gen Genet. 1982;185(2):223–228. doi: 10.1007/BF00330791. [DOI] [PubMed] [Google Scholar]
- Kieser T., Melton R. E. Plasmid pIJ699, a multi-copy positive-selection vector for Streptomyces. Gene. 1988 May 15;65(1):83–91. doi: 10.1016/0378-1119(88)90419-2. [DOI] [PubMed] [Google Scholar]
- Lomovskaya N. D., Mkrtumian N. M., Gostimskaya N. L., Danilenko V. N. Characterization of temperate actinophage phi C31 isolated from Streptomyces coelicolor A3(2). J Virol. 1972 Feb;9(2):258–262. doi: 10.1128/jvi.9.2.258-262.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lydiate D. J., Malpartida F., Hopwood D. A. The Streptomyces plasmid SCP2*: its functional analysis and development into useful cloning vectors. Gene. 1985;35(3):223–235. doi: 10.1016/0378-1119(85)90001-0. [DOI] [PubMed] [Google Scholar]
- MacNeil D. J., Gewain K. M., Ruby C. L., Dezeny G., Gibbons P. H., MacNeil T. Analysis of Streptomyces avermitilis genes required for avermectin biosynthesis utilizing a novel integration vector. Gene. 1992 Feb 1;111(1):61–68. doi: 10.1016/0378-1119(92)90603-m. [DOI] [PubMed] [Google Scholar]
- Rodicio M. R., Bruton C. J., Chater K. F. New derivatives of the Streptomyces temperate phage phi C31 useful for the cloning and functional analysis of Streptomyces DNA. Gene. 1985;34(2-3):283–292. doi: 10.1016/0378-1119(85)90137-4. [DOI] [PubMed] [Google Scholar]
- Sang G. W., Zhang Y. G., Shi Q. X., Shen K. Y., Lu F. Y., Zhao X. J., Wang M. Q., Liu X. L., Yuan Y. Y. [Chronic toxicity of gossypol and the relationship to its metabolic fate in dogs and monkeys (author's transl)]. Zhongguo Yao Li Xue Bao. 1980 Sep;1(1):39–43. [PubMed] [Google Scholar]
- Schauer A. T., Nelson A. D., Daniel J. B. Tn4563 transposition in Streptomyces coelicolor and its application to isolation of new morphological mutants. J Bacteriol. 1991 Aug;173(16):5060–5067. doi: 10.1128/jb.173.16.5060-5067.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smokvina T., Mazodier P., Boccard F., Thompson C. J., Guérineau M. Construction of a series of pSAM2-based integrative vectors for use in actinomycetes. Gene. 1990 Sep 28;94(1):53–59. doi: 10.1016/0378-1119(90)90467-6. [DOI] [PubMed] [Google Scholar]
- Suami T., Ogawa S., Chida N. The revised structure of validamycin A. J Antibiot (Tokyo) 1980 Jan;33(1):98–99. doi: 10.7164/antibiotics.33.98. [DOI] [PubMed] [Google Scholar]
- Thompson C. J., Kieser T., Ward J. M., Hopwood D. A. Physical analysis of antibiotic-resistance genes from Streptomyces and their use in vector construction. Gene. 1982 Nov;20(1):51–62. doi: 10.1016/0378-1119(82)90086-5. [DOI] [PubMed] [Google Scholar]
- Zhou X. F., Zhou Q. Interspecific protoplast fusion of Streptomyces hygroscopicus var. yingchengenisis with Streptomyces qingfengmyceticus and biological characterization of their recombinants. Chin J Biotechnol. 1989;5(3):161–166. [PubMed] [Google Scholar]