Abstract
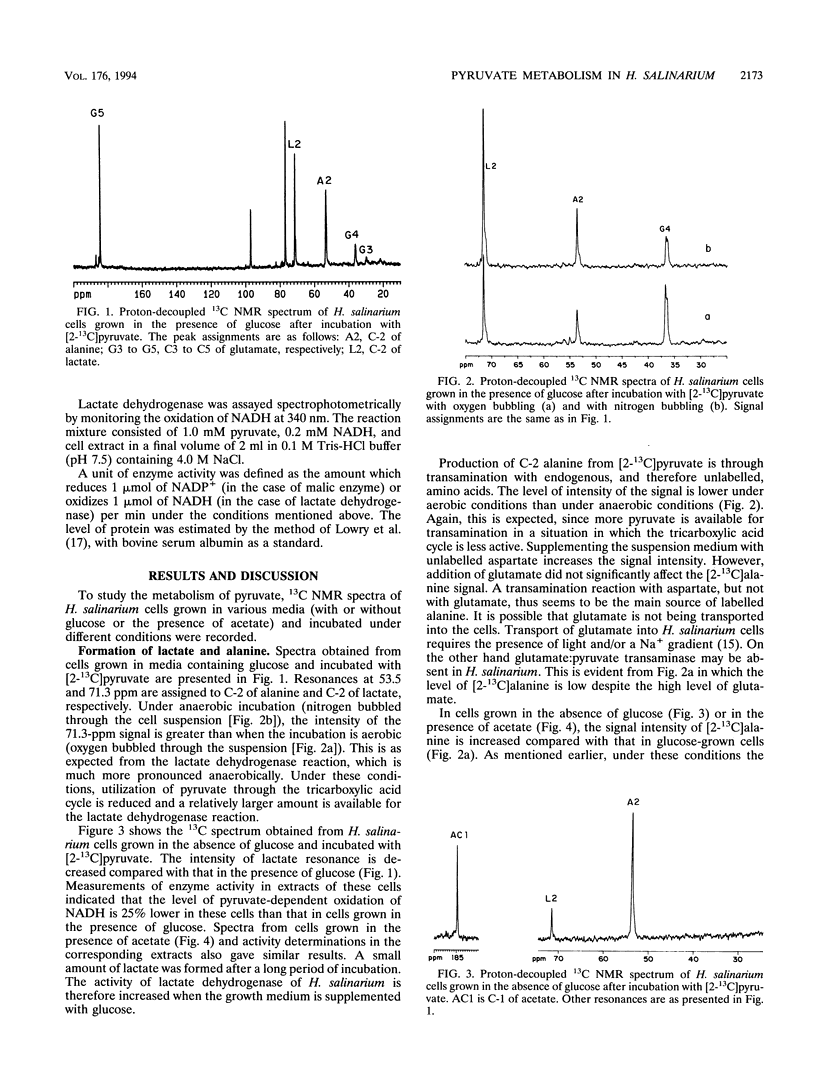
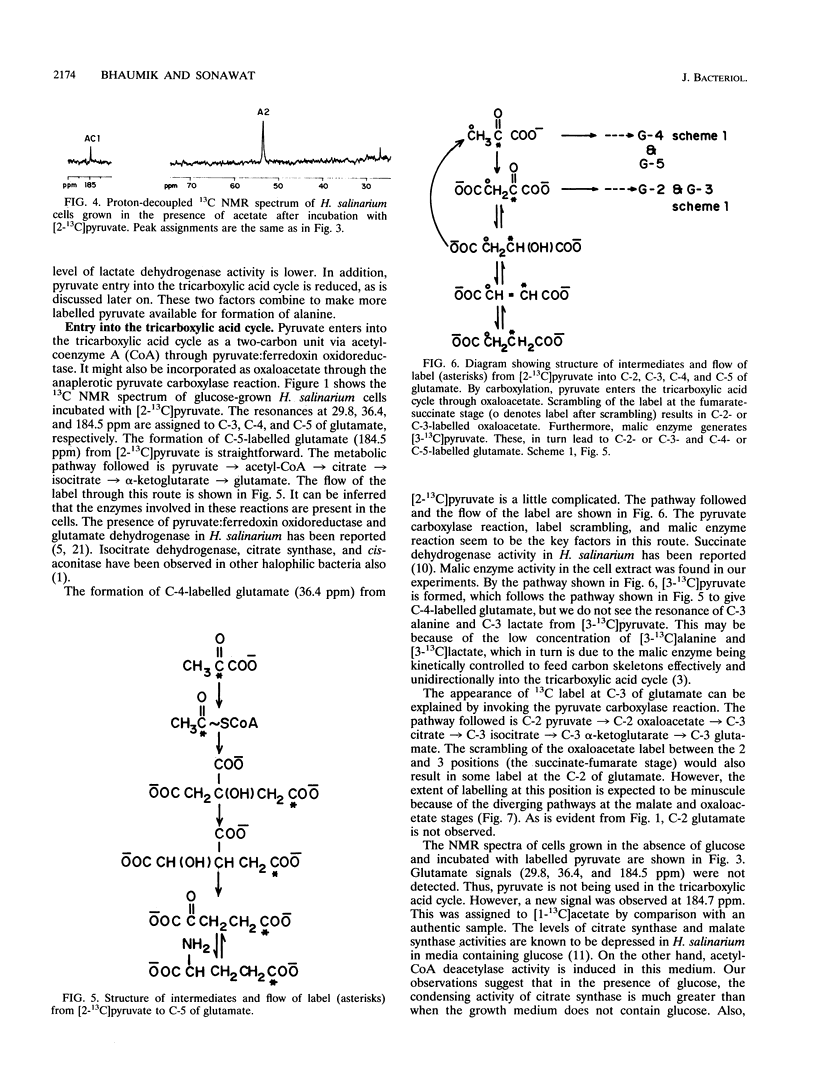
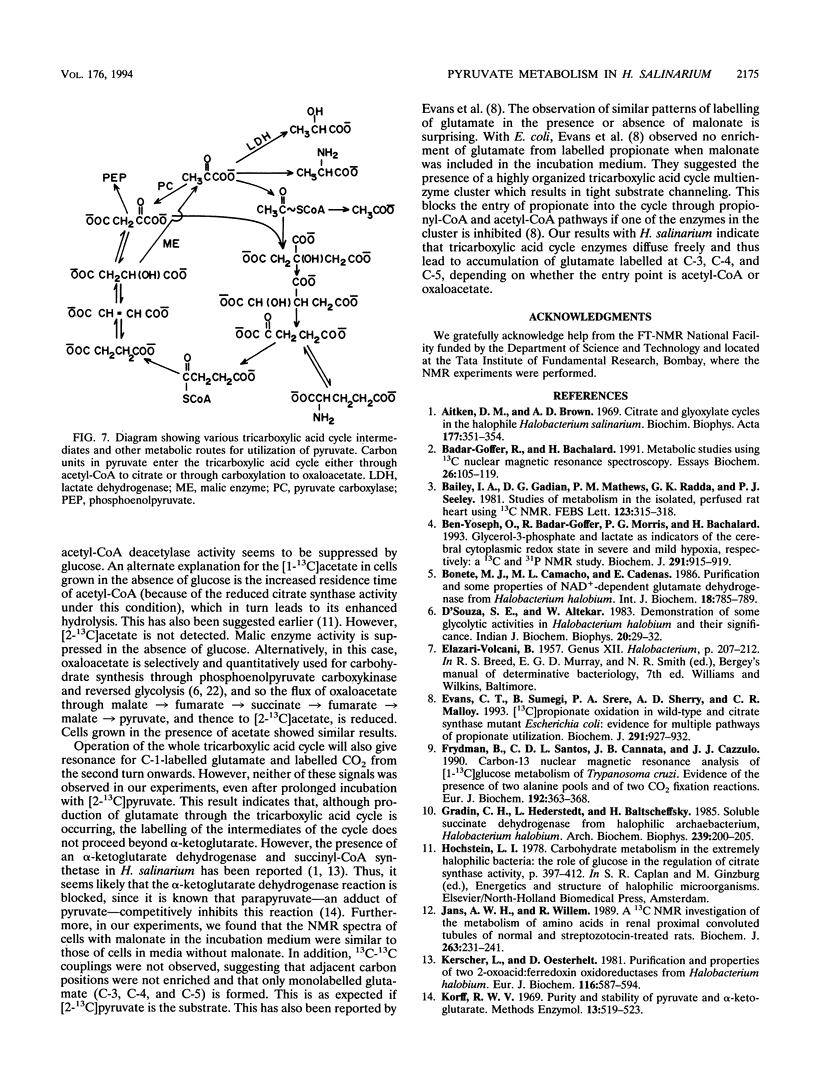
13C nuclear magnetic resonance spectroscopy was used to study the metabolism of [2-13C]pyruvate in intact cells of Halobacterium salinarium. The spectra of these cells show that pyruvate is reduced to lactic acid and transaminated to alanine. The intensity of C-2 lactate is higher under anaerobic conditions than under aerobic conditions. When cells are grown in the absence of glucose, the level of C-2 lactate intensity is lower. In extracts of these cells, the level of NADH-dependent lactate dehydrogenase activity is lower than that of cells grown in the presence of glucose. A C-5 glutamate resonance suggests the entry of pyruvate into the tricarboxylic acid cycle through acetyl-coenzyme A. In addition, the label is also observed at C-3 and C-4 of glutamate, signifying a pyruvate carboxylase-type reaction and scrambling of label at the fumarate-succinate stage plus malic enzyme operation, respectively. Citrate synthase and malic enzyme activity appear to be controlled by the growth conditions of H. salinarium.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aitken D. M., Brown A. D. Citrate and glyoxylate cycles in the halophil, Halobacterium salinarium. Biochim Biophys Acta. 1969 Apr 1;177(2):351–354. doi: 10.1016/0304-4165(69)90148-2. [DOI] [PubMed] [Google Scholar]
- Badar-Goffer R., Bachelard H. Metabolic studies using 13C nuclear magnetic resonance spectroscopy. Essays Biochem. 1991;26:105–119. [PubMed] [Google Scholar]
- Bailey I. A., Gadian D. G., Matthews P. M., Radda G. K., Seeley P. J. Studies of metabolism in the isolated, perfused rat heart using 13C NMR. FEBS Lett. 1981 Jan 26;123(2):315–318. doi: 10.1016/0014-5793(81)80317-1. [DOI] [PubMed] [Google Scholar]
- Ben-Yoseph O., Badar-Goffer R. S., Morris P. G., Bachelard H. S. Glycerol 3-phosphate and lactate as indicators of the cerebral cytoplasmic redox state in severe and mild hypoxia respectively: a 13C- and 31P-n.m.r. study. Biochem J. 1993 May 1;291(Pt 3):915–919. doi: 10.1042/bj2910915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Souza S. E., Altekar W. Demonstration of some glycolytic activities in Halobacterium halobium & their significance. Indian J Biochem Biophys. 1983 Feb;20(1):29–32. [PubMed] [Google Scholar]
- Evans C. T., Sumegi B., Srere P. A., Sherry A. D., Malloy C. R. [13C]propionate oxidation in wild-type and citrate synthase mutant Escherichia coli: evidence for multiple pathways of propionate utilization. Biochem J. 1993 May 1;291(Pt 3):927–932. doi: 10.1042/bj2910927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frydman B., de los Santos C., Cannata J. J., Cazzulo J. J. Carbon-13 nuclear magnetic resonance analysis of [1-13C]glucose metabolism in Trypanosoma cruzi. Evidence of the presence of two alanine pools and of two CO2 fixation reactions. Eur J Biochem. 1990 Sep 11;192(2):363–368. doi: 10.1111/j.1432-1033.1990.tb19235.x. [DOI] [PubMed] [Google Scholar]
- Gradin C. H., Hederstedt L., Baltscheffsky H. Soluble succinate dehydrogenase from the halophilic archaebacterium, Halobacterium halobium. Arch Biochem Biophys. 1985 May 15;239(1):200–205. doi: 10.1016/0003-9861(85)90827-6. [DOI] [PubMed] [Google Scholar]
- Jans A. W., Willem R. A 13C-n.m.r. investigation of the metabolism of amino acids in renal proximal convoluted tubules of normal and streptozotocin-treated rats and rabbits. Biochem J. 1989 Oct 1;263(1):231–241. doi: 10.1042/bj2630231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerscher L., Oesterhelt D. Purification and properties of two 2-oxoacid:ferredoxin oxidoreductases from Halobacterium halobium. Eur J Biochem. 1981 Jun 1;116(3):587–594. doi: 10.1111/j.1432-1033.1981.tb05376.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Malloy C. R., Sherry A. D., Jeffrey F. M. Analysis of tricarboxylic acid cycle of the heart using 13C isotope isomers. Am J Physiol. 1990 Sep;259(3 Pt 2):H987–H995. doi: 10.1152/ajpheart.1990.259.3.H987. [DOI] [PubMed] [Google Scholar]
- Narbad A., Hewlins M. J., Gacesa P., Russell N. J. The use of 13C-n.m.r. spectroscopy to monitor alginate biosynthesis in mucoid Pseudomonas aeruginosa. Biochem J. 1990 May 1;267(3):579–584. doi: 10.1042/bj2670579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaga W., Lottspeich F., Oesterhelt D. Improved purification, crystallization and primary structure of pyruvate:ferredoxin oxidoreductase from Halobacterium halobium. Eur J Biochem. 1992 Apr 1;205(1):391–397. doi: 10.1111/j.1432-1033.1992.tb16792.x. [DOI] [PubMed] [Google Scholar]
- Rawal N., Kelkar S. M., Altekar W. Alternative routes of carbohydrate metabolism in halophilic archaebacteria. Indian J Biochem Biophys. 1988 Dec;25(6):674–686. [PubMed] [Google Scholar]
- Sonawat H. M., Srivastava S., Swaminathan S., Govil G. Glycolysis and Entner-Doudoroff pathways in Halobacterium halobium: some new observations based on 13C NMR spectroscopy. Biochem Biophys Res Commun. 1990 Nov 30;173(1):358–362. doi: 10.1016/s0006-291x(05)81065-4. [DOI] [PubMed] [Google Scholar]



