Abstract
Type 1 and F1C fimbriae are surface organelles of Escherichia coli which mediate receptor-specific binding to different host surfaces. Such fimbriae are found on strains associated with urinary tract infections. The specific receptor binding of the fimbriae is due to the presence of receptor recognition proteins present in the organelles as minor structural elements. The organization of the fim and foc gene clusters encoding these fimbriae, as well as the structures of the organelles, are very similar, although the actual sequence homology of the structural elements is not remarkable; notably, the sequence identity between the minor components of the type 1 and F1C fimbriae is only 34 to 41%. Type 1 fimbriae mediate agglutination of guinea pig erythrocytes, whereas F1C fimbriae do not confer agglutination of any types of erythrocytes tested. However, F1C fimbriae mediate specific adhesion to epithelial cells in the collecting ducts of the human kidney as well as to cells of various cell lines. This report addresses the question of fimbrial promiscuity. Our data indicate that minor fimbrial structural elements can be exchanged between the two fimbrial systems, resulting in hybrid organelles with changed receptor specificity. This is the first study on reciprocal exchange of structural components from two different fimbrial systems.
Full text
PDF
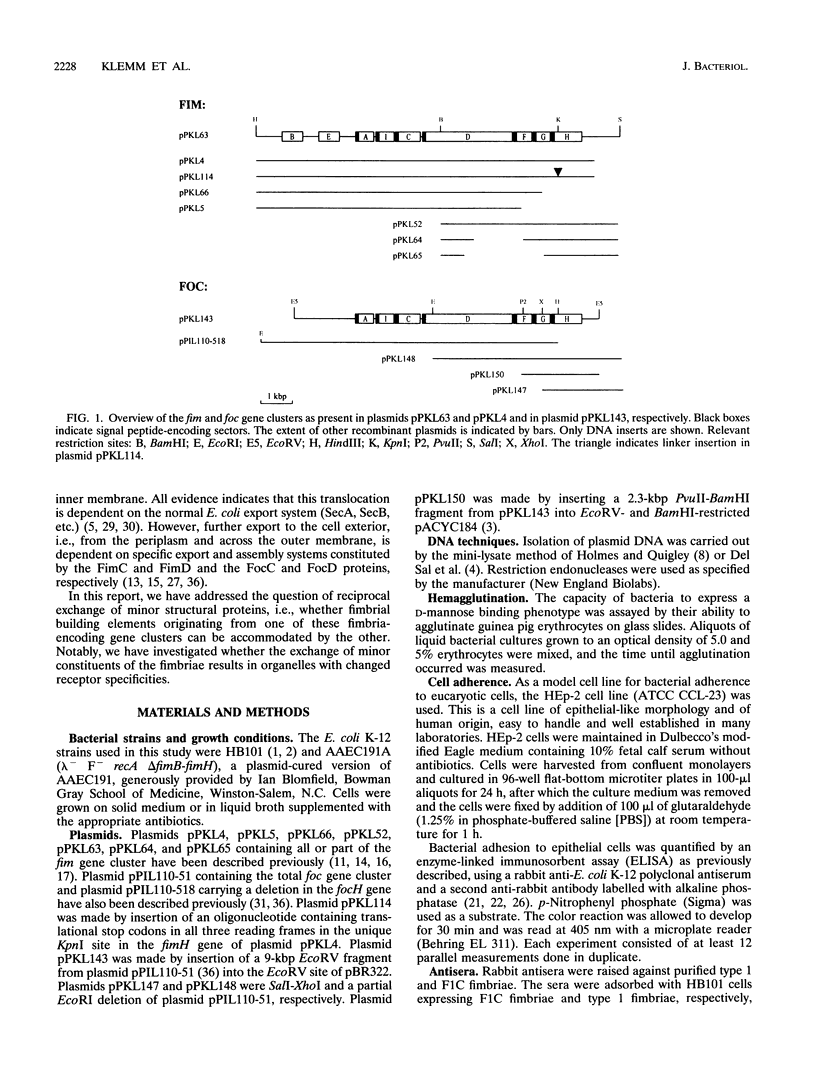



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blomfield I. C., Vaughn V., Rest R. F., Eisenstein B. I. Allelic exchange in Escherichia coli using the Bacillus subtilis sacB gene and a temperature-sensitive pSC101 replicon. Mol Microbiol. 1991 Jun;5(6):1447–1457. doi: 10.1111/j.1365-2958.1991.tb00791.x. [DOI] [PubMed] [Google Scholar]
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Chang A. C., Cohen S. N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978 Jun;134(3):1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Sal G., Manfioletti G., Schneider C. The CTAB-DNA precipitation method: a common mini-scale preparation of template DNA from phagemids, phages or plasmids suitable for sequencing. Biotechniques. 1989 May;7(5):514–520. [PubMed] [Google Scholar]
- Dodd D. C., Bassford P. J., Jr, Eisenstein B. I. Dependence of secretion and assembly of type 1 fimbrial subunits of Escherichia coli on normal protein export. J Bacteriol. 1984 Sep;159(3):1077–1079. doi: 10.1128/jb.159.3.1077-1079.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita K., Yamamoto T., Yokota T., Kitagawa R. In vitro adherence of type 1-fimbriated uropathogenic Escherichia coli to human ureteral mucosa. Infect Immun. 1989 Aug;57(8):2574–2579. doi: 10.1128/iai.57.8.2574-2579.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes D. S., Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981 Jun;114(1):193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- Hull R., Bieler S., Falkow S., Hull S. Chromosomal map position of genes encoding P adhesins in uropathogenic Escherichia coli. Infect Immun. 1986 Feb;51(2):693–695. doi: 10.1128/iai.51.2.693-695.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemm P., Christiansen G. The fimD gene required for cell surface localization of Escherichia coli type 1 fimbriae. Mol Gen Genet. 1990 Jan;220(2):334–338. doi: 10.1007/BF00260505. [DOI] [PubMed] [Google Scholar]
- Klemm P., Christiansen G. Three fim genes required for the regulation of length and mediation of adhesion of Escherichia coli type 1 fimbriae. Mol Gen Genet. 1987 Jul;208(3):439–445. doi: 10.1007/BF00328136. [DOI] [PubMed] [Google Scholar]
- Klemm P. FimC, a chaperone-like periplasmic protein of Escherichia coli involved in biogenesis of type 1 fimbriae. Res Microbiol. 1992 Nov-Dec;143(9):831–838. doi: 10.1016/0923-2508(92)90070-5. [DOI] [PubMed] [Google Scholar]
- Klemm P. Fimbrial adhesions of Escherichia coli. Rev Infect Dis. 1985 May-Jun;7(3):321–340. doi: 10.1093/clinids/7.3.321. [DOI] [PubMed] [Google Scholar]
- Klemm P., Jørgensen B. J., van Die I., de Ree H., Bergmans H. The fim genes responsible for synthesis of type 1 fimbriae in Escherichia coli, cloning and genetic organization. Mol Gen Genet. 1985;199(3):410–414. doi: 10.1007/BF00330751. [DOI] [PubMed] [Google Scholar]
- Klemm P., Krogfelt K. A., Hedegaard L., Christiansen G. The major subunit of Escherichia coli type 1 fimbriae is not required for D-mannose-specific adhesion. Mol Microbiol. 1990 Apr;4(4):553–559. doi: 10.1111/j.1365-2958.1990.tb00623.x. [DOI] [PubMed] [Google Scholar]
- Klemm P., Orskov I., Orskov F. F7 and type 1-like fimbriae from three Escherichia coli strains isolated from urinary tract infections: protein chemical and immunological aspects. Infect Immun. 1982 May;36(2):462–468. doi: 10.1128/iai.36.2.462-468.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemm P. The fimA gene encoding the type-1 fimbrial subunit of Escherichia coli. Nucleotide sequence and primary structure of the protein. Eur J Biochem. 1984 Sep 3;143(2):395–399. doi: 10.1111/j.1432-1033.1984.tb08386.x. [DOI] [PubMed] [Google Scholar]
- Korhonen T. K., Valtonen M. V., Parkkinen J., Väisänen-Rhen V., Finne J., Orskov F., Orskov I., Svenson S. B., Mäkelä P. H. Serotypes, hemolysin production, and receptor recognition of Escherichia coli strains associated with neonatal sepsis and meningitis. Infect Immun. 1985 May;48(2):486–491. doi: 10.1128/iai.48.2.486-491.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korhonen T. K., Väisänen-Rhen V., Rhen M., Pere A., Parkkinen J., Finne J. Escherichia coli fimbriae recognizing sialyl galactosides. J Bacteriol. 1984 Aug;159(2):762–766. doi: 10.1128/jb.159.2.762-766.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreft B., Carstensen O., Straube E., Bohnet S., Hacker J., Marre R. Adherence to and cytotoxicity of Escherichia coli for eucaryotic cell lines quantified by MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide). Zentralbl Bakteriol. 1992 Jan;276(2):231–242. doi: 10.1016/s0934-8840(11)80010-1. [DOI] [PubMed] [Google Scholar]
- Kreft B., Marre R., Schramm U., Wirth R. Aggregation substance of Enterococcus faecalis mediates adhesion to cultured renal tubular cells. Infect Immun. 1992 Jan;60(1):25–30. doi: 10.1128/iai.60.1.25-30.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krogfelt K. A. Bacterial adhesion: genetics, biogenesis, and role in pathogenesis of fimbrial adhesins of Escherichia coli. Rev Infect Dis. 1991 Jul-Aug;13(4):721–735. doi: 10.1093/clinids/13.4.721. [DOI] [PubMed] [Google Scholar]
- Krogfelt K. A., Bergmans H., Klemm P. Direct evidence that the FimH protein is the mannose-specific adhesin of Escherichia coli type 1 fimbriae. Infect Immun. 1990 Jun;58(6):1995–1998. doi: 10.1128/iai.58.6.1995-1998.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krogfelt K. A., Klemm P. Investigation of minor components of Escherichia coli type 1 fimbriae: protein chemical and immunological aspects. Microb Pathog. 1988 Mar;4(3):231–238. doi: 10.1016/0882-4010(88)90073-3. [DOI] [PubMed] [Google Scholar]
- Marre R., Kreft B., Hacker J. Genetically engineered S and F1C fimbriae differ in their contribution to adherence of Escherichia coli to cultured renal tubular cells. Infect Immun. 1990 Oct;58(10):3434–3437. doi: 10.1128/iai.58.10.3434-3437.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orskov I., Orskov F., Birch-Andersen A. Comparison of Escherichia coli fimbrial antigen F7 with type 1 fimbriae. Infect Immun. 1980 Feb;27(2):657–666. doi: 10.1128/iai.27.2.657-666.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott M., Hoschützky H., Jann K., Van Die I., Hacker J. Gene clusters for S fimbrial adhesin (sfa) and F1C fimbriae (foc) of Escherichia coli: comparative aspects of structure and function. J Bacteriol. 1988 Sep;170(9):3983–3990. doi: 10.1128/jb.170.9.3983-3990.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugsley A. P. The complete general secretory pathway in gram-negative bacteria. Microbiol Rev. 1993 Mar;57(1):50–108. doi: 10.1128/mr.57.1.50-108.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall L. L., Hardy S. J. Export of protein in bacteria. Microbiol Rev. 1984 Dec;48(4):290–298. doi: 10.1128/mr.48.4.290-298.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riegman N., Kusters R., Van Veggel H., Bergmans H., Van Bergen en Henegouwen P., Hacker J., Van Die I. F1C fimbriae of a uropathogenic Escherichia coli strain: genetic and functional organization of the foc gene cluster and identification of minor subunits. J Bacteriol. 1990 Feb;172(2):1114–1120. doi: 10.1128/jb.172.2.1114-1120.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell P. W., Orndorff P. E. Lesions in two Escherichia coli type 1 pilus genes alter pilus number and length without affecting receptor binding. J Bacteriol. 1992 Sep;174(18):5923–5935. doi: 10.1128/jb.174.18.5923-5935.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salit I. E., Gotschlich E. C. Type I Escherichia coli pili: characterization of binding to monkey kidney cells. J Exp Med. 1977 Nov 1;146(5):1182–1194. doi: 10.1084/jem.146.5.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmoll T., Hoschützky H., Morschhäuser J., Lottspeich F., Jann K., Hacker J. Analysis of genes coding for the sialic acid-binding adhesin and two other minor fimbrial subunits of the S-fimbrial adhesin determinant of Escherichia coli. Mol Microbiol. 1989 Dec;3(12):1735–1744. doi: 10.1111/j.1365-2958.1989.tb00159.x. [DOI] [PubMed] [Google Scholar]
- Virkola R., Westerlund B., Holthöfer H., Parkkinen J., Kekomäki M., Korhonen T. K. Binding characteristics of Escherichia coli adhesins in human urinary bladder. Infect Immun. 1988 Oct;56(10):2615–2622. doi: 10.1128/iai.56.10.2615-2622.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Die I., Kramer C., Hacker J., Bergmans H., Jongen W., Hoekstra W. Nucleotide sequence of the genes coding for minor fimbrial subunits of the F1C fimbriae of Escherichia coli. Res Microbiol. 1991 Jul-Aug;142(6):653–658. doi: 10.1016/0923-2508(91)90078-o. [DOI] [PubMed] [Google Scholar]
- van Die I., van Geffen B., Hoekstra W., Bergmans H. Type 1C fimbriae of a uropathogenic Escherichia coli strain: cloning and characterization of the genes involved in the expression of the 1C antigen and nucleotide sequence of the subunit gene. Gene. 1985;34(2-3):187–196. doi: 10.1016/0378-1119(85)90127-1. [DOI] [PubMed] [Google Scholar]