Abstract
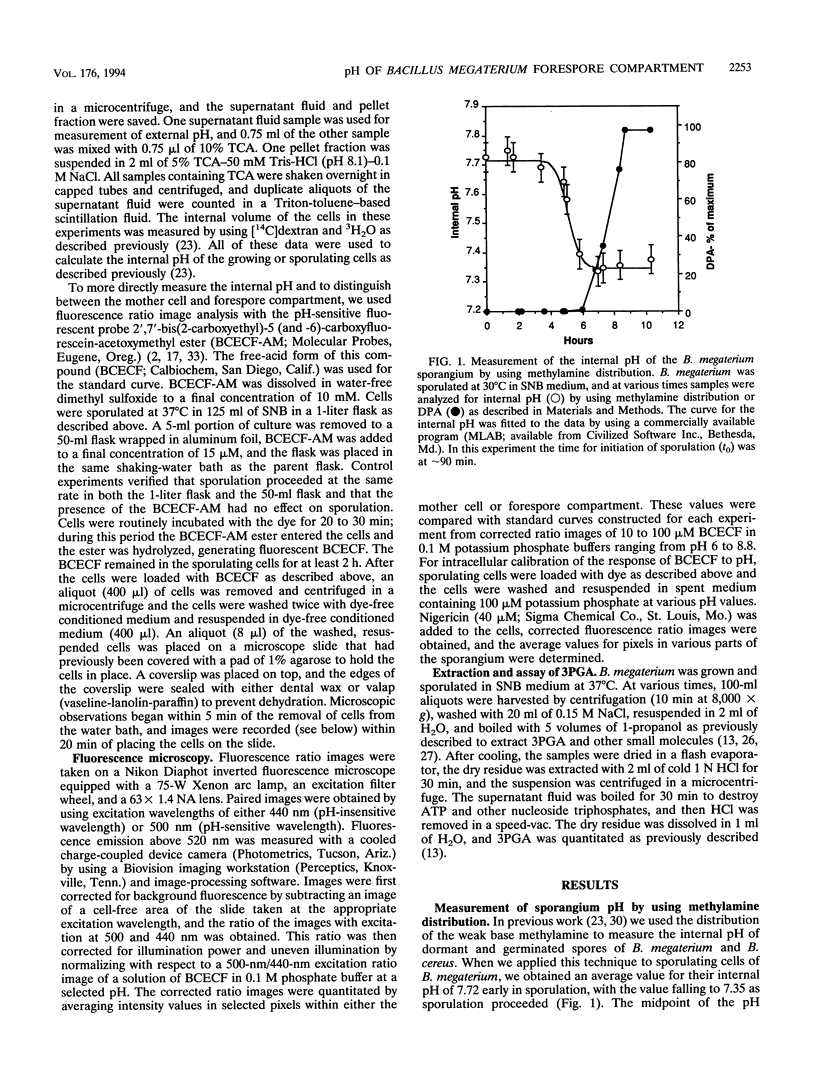
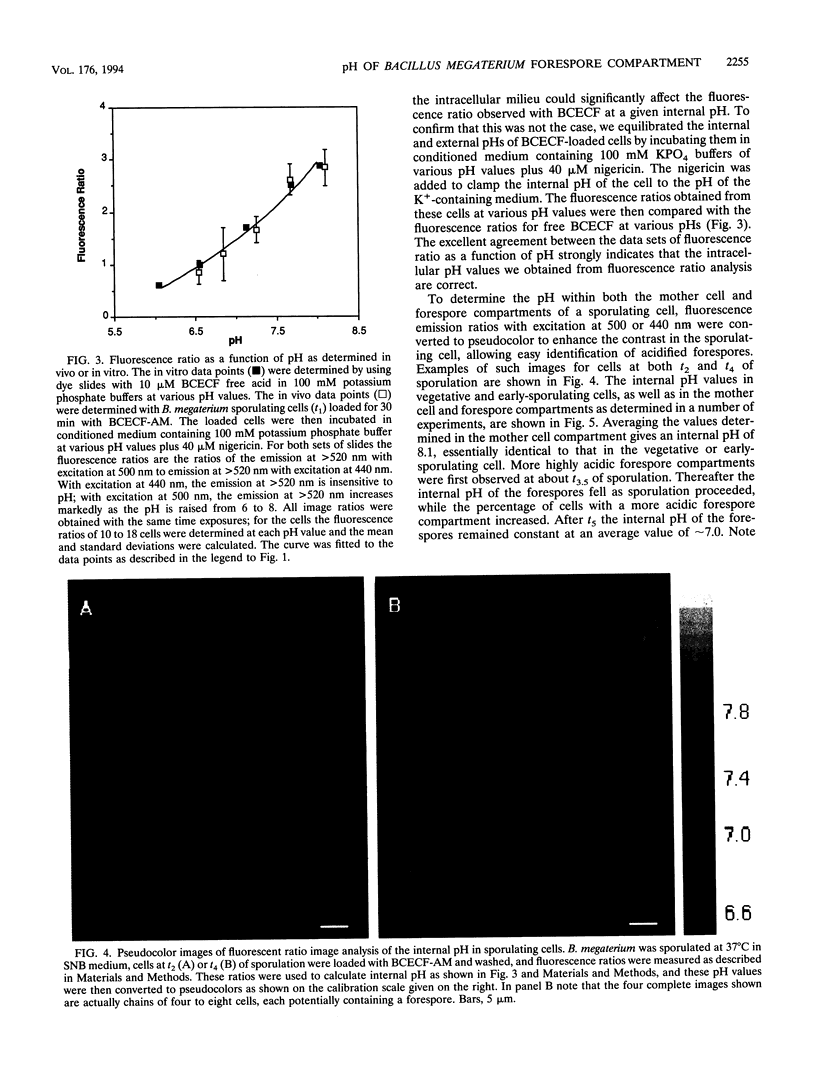
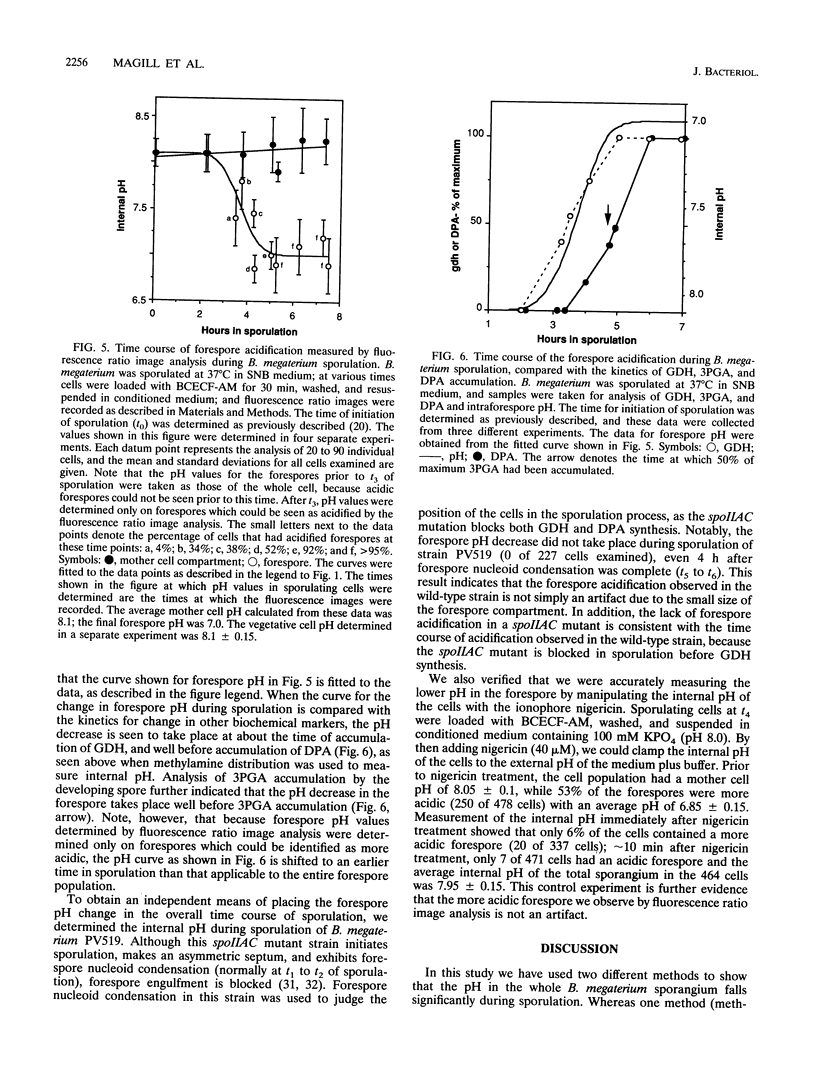
Previous work has shown that the internal pH of dormant spores of Bacillus species is more than 1 pH U below that of growing cells but rises to that of growing cells in the first minutes of spore germination. In the present work the internal pH of the whole Bacillus megaterium sporangium was measured by the distribution of the weak base methylamine and was found to decrease by approximately 0.4 during sporulation. By using fluorescence ratio image analysis with a fluorescein derivative, 2',7'-bis(2-carboxyethyl)-5 (and -6)-carboxyfluorescein (BCECF), whose fluorescence is pH sensitive, the internal pH of the mother cell was found to remain constant during sporulation at a value of 8.1, similar to that in the vegetative cell. Whereas the internal pH of the forespore was initially approximately 8.1, this value fell to approximately 7.0 approximately 90 min before synthesis of dipicolinic acid and well before accumulation of the depot of 3-phosphoglyceric acid. The pH in the forespore compartment was brought to that of the mother cell by suspending sporulating cells in a pH 8 potassium phosphate buffer plus the ionophore nigericin to clamp the internal pH of the cells to that of the external medium. We suggest that at a minimum, acidification of the forespore may regulate the activity of phosphoglycerate mutase, which is the enzyme known to be regulated to allow 3-phosphoglyceric acid accumulation during sporulation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barton J. K., den Hollander J. A., Lee T. M., MacLaughlin A., Shulman R. G. Measurement of the internal pH of yeast spores by 31P nuclear magnetic resonance. Proc Natl Acad Sci U S A. 1980 May;77(5):2470–2473. doi: 10.1073/pnas.77.5.2470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bright G. R., Fisher G. W., Rogowska J., Taylor D. L. Fluorescence ratio imaging microscopy: temporal and spatial measurements of cytoplasmic pH. J Cell Biol. 1987 Apr;104(4):1019–1033. doi: 10.1083/jcb.104.4.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker S. J., Lang D. R. Membrane bioenergetic parameters in uncoupler-resistant mutants of Bacillus megaterium. J Biol Chem. 1978 Oct 10;253(19):6738–6743. [PubMed] [Google Scholar]
- Errington J. Bacillus subtilis sporulation: regulation of gene expression and control of morphogenesis. Microbiol Rev. 1993 Mar;57(1):1–33. doi: 10.1128/mr.57.1.1-33.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gow N. A., Kropf D. L., Harold F. M. Growing hyphae of Achlya bisexualis generate a longitudinal pH gradient in the surrounding medium. J Gen Microbiol. 1984 Nov;130(11):2967–2974. doi: 10.1099/00221287-130-11-2967. [DOI] [PubMed] [Google Scholar]
- Gross J. D., Bradbury J., Kay R. R., Peacey M. J. Intracellular pH and the control of cell differentiation in Dictyostelium discoideum. Nature. 1983 May 19;303(5914):244–245. doi: 10.1038/303244a0. [DOI] [PubMed] [Google Scholar]
- Harold R. L., Harold F. M. Ionophores and cytochalasins modulate branching in Achlya bisexualis. J Gen Microbiol. 1986 Jan;132(1):213–219. doi: 10.1099/00221287-132-1-213. [DOI] [PubMed] [Google Scholar]
- Inouye K. Measurements of intracellular pH and its relevance to cell differentiation in Dictyostelium discoideum. J Cell Sci. 1985 Jun;76:235–245. doi: 10.1242/jcs.76.1.235. [DOI] [PubMed] [Google Scholar]
- Jamieson G. A., Jr, Frazier W. A., Schlesinger P. H. Transient increase in intracellular pH during Dictyostelium differentiation. J Cell Biol. 1984 Nov;99(5):1883–1887. doi: 10.1083/jcb.99.5.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krulwich T. A., Guffanti A. A. Physiology of acidophilic and alkalophilic bacteria. Adv Microb Physiol. 1983;24:173–214. doi: 10.1016/s0065-2911(08)60386-0. [DOI] [PubMed] [Google Scholar]
- Kuhn N. J., Setlow B., Setlow P. Manganese(II) activation of 3-phosphoglycerate mutase of Bacillus megaterium: pH-sensitive interconversion of active and inactive forms. Arch Biochem Biophys. 1993 Nov 1;306(2):342–349. doi: 10.1006/abbi.1993.1521. [DOI] [PubMed] [Google Scholar]
- Loshon C. A., Setlow P. Levels of small molecules in dormant spores of Sporosarcina species and comparison with levels in spores of Bacillus and Clostridium species. Can J Microbiol. 1993 Feb;39(2):259–262. doi: 10.1139/m93-036. [DOI] [PubMed] [Google Scholar]
- Molenaar D., Abee T., Konings W. N. Continuous measurement of the cytoplasmic pH in Lactococcus lactis with a fluorescent pH indicator. Biochim Biophys Acta. 1991 Nov 14;1115(1):75–83. doi: 10.1016/0304-4165(91)90014-8. [DOI] [PubMed] [Google Scholar]
- Padan E., Zilberstein D., Schuldiner S. pH homeostasis in bacteria. Biochim Biophys Acta. 1981 Dec;650(2-3):151–166. doi: 10.1016/0304-4157(81)90004-6. [DOI] [PubMed] [Google Scholar]
- Poolman B., Nijssen R. M., Konings W. N. Dependence of Streptococcus lactis phosphate transport on internal phosphate concentration and internal pH. J Bacteriol. 1987 Dec;169(12):5373–5378. doi: 10.1128/jb.169.12.5373-5378.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rink T. J., Tsien R. Y., Pozzan T. Cytoplasmic pH and free Mg2+ in lymphocytes. J Cell Biol. 1982 Oct;95(1):189–196. doi: 10.1083/jcb.95.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roncal T., Ugalde U. O., Irastorza A. Calcium-induced conidiation in Penicillium cyclopium: calcium triggers cytosolic alkalinization at the hyphal tip. J Bacteriol. 1993 Feb;175(3):879–886. doi: 10.1128/jb.175.3.879-886.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotman Y., Fields M. L. A modified reagent for dipicolinic acid analysis. Anal Biochem. 1968 Jan;22(1):168–168. doi: 10.1016/0003-2697(68)90272-8. [DOI] [PubMed] [Google Scholar]
- Sanchez-Salas J. L., Santiago-Lara M. L., Setlow B., Sussman M. D., Setlow P. Properties of Bacillus megaterium and Bacillus subtilis mutants which lack the protease that degrades small, acid-soluble proteins during spore germination. J Bacteriol. 1992 Feb;174(3):807–814. doi: 10.1128/jb.174.3.807-814.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow B., Magill N., Febbroriello P., Nakhimovsky L., Koppel D. E., Setlow P. Condensation of the forespore nucleoid early in sporulation of Bacillus species. J Bacteriol. 1991 Oct;173(19):6270–6278. doi: 10.1128/jb.173.19.6270-6278.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow B., Setlow P. Measurements of the pH within dormant and germinated bacterial spores. Proc Natl Acad Sci U S A. 1980 May;77(5):2474–2476. doi: 10.1073/pnas.77.5.2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow J. K., Randesi M., Adams J. G., Setlow B., Setlow P. Mutation and killing of Escherichia coli expressing a cloned Bacillus subtilis gene whose product alters DNA conformation. J Bacteriol. 1992 May;174(9):2943–2950. doi: 10.1128/jb.174.9.2943-2950.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow P., Kornberg A. Biochemical studies of bacterial sporulation and germination. XXII. Energy metabolism in early stages of germination of Bacillus megaterium spores. J Biol Chem. 1970 Jul 25;245(14):3637–3644. [PubMed] [Google Scholar]
- Singh R. P., Setlow B., Setlow P. Levels of small molecules and enzymes in the mother cell compartment and the forespore of sporulating Bacillus megaterium. J Bacteriol. 1977 Jun;130(3):1130–1138. doi: 10.1128/jb.130.3.1130-1138.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R. P., Setlow P. Purification and properties of phosphoglycerate phosphomutase from spores and cells of Bacillus megaterium. J Bacteriol. 1979 Feb;137(2):1024–1027. doi: 10.1128/jb.137.2.1024-1027.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R. P., Setlow P. Regulation of phosphoglycerate phosphomutase in developing forespores and dormant and germinated spores of Bacillus megaterium by the level of free manganous ions. J Bacteriol. 1979 Sep;139(3):889–898. doi: 10.1128/jb.139.3.889-898.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow B. M., Setlow B., Setlow P. Levels of H+ and other monovalent cations in dormant and germinating spores of Bacillus megaterium. J Bacteriol. 1981 Oct;148(1):20–29. doi: 10.1128/jb.148.1.20-29.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao Y. P., Hudspeth D. S., Vary P. S. Cloning and sequencing of the Bacillus megaterium spoIIA operon. Biochimie. 1992 Jul-Aug;74(7-8):695–704. doi: 10.1016/0300-9084(92)90142-2. [DOI] [PubMed] [Google Scholar]
- Tsujimoto K., Semadeni M., Huflejt M., Packer L. Intracellular pH of halobacteria can be determined by the fluorescent dye 2', 7'-bis(carboxyethyl)-5(6)-carboxyfluorescein. Biochem Biophys Res Commun. 1988 Aug 30;155(1):123–129. doi: 10.1016/s0006-291x(88)81058-1. [DOI] [PubMed] [Google Scholar]
- Watabe K., Freese E. Purification and properties of the manganese-dependent phosphoglycerate mutase of Bacillus subtilis. J Bacteriol. 1979 Feb;137(2):773–778. doi: 10.1128/jb.137.2.773-778.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]