Abstract
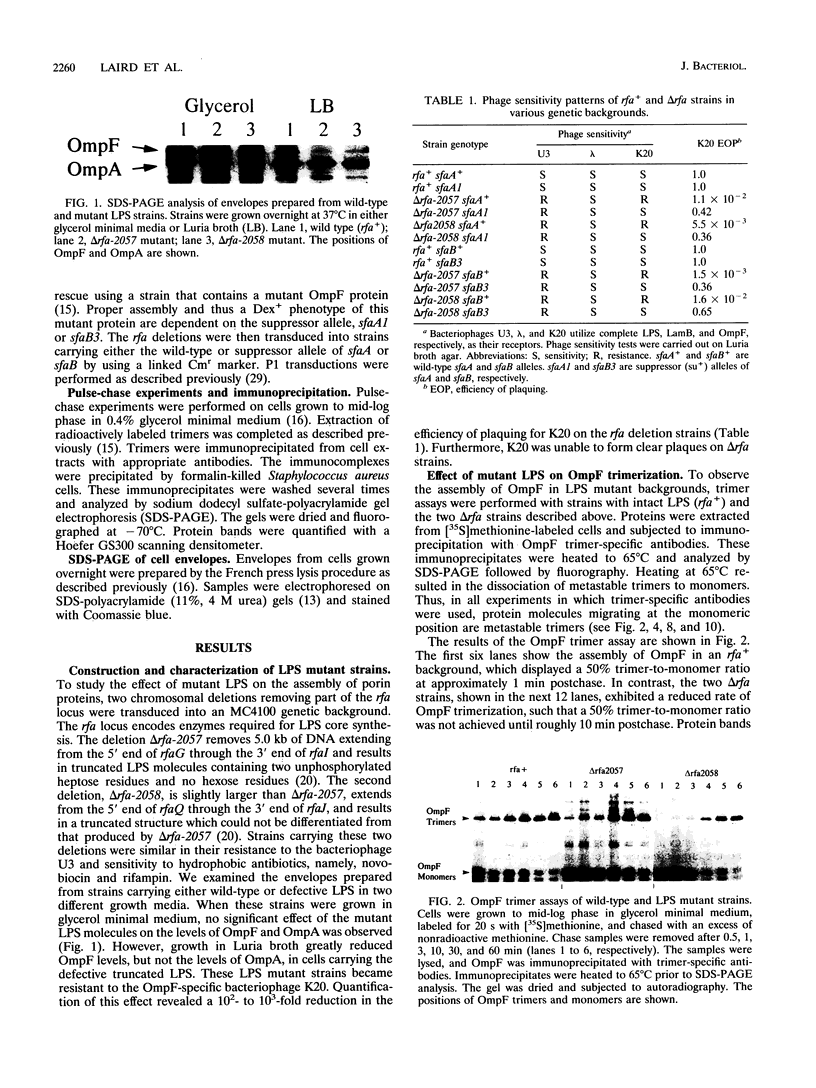
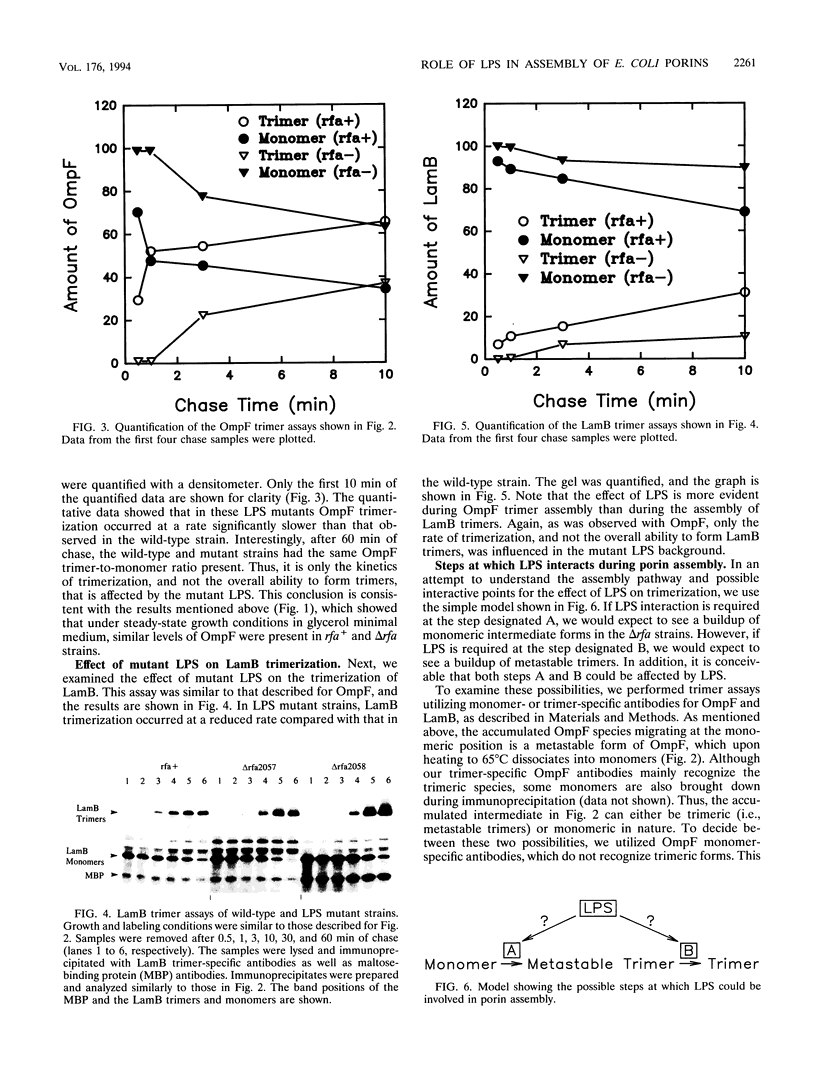
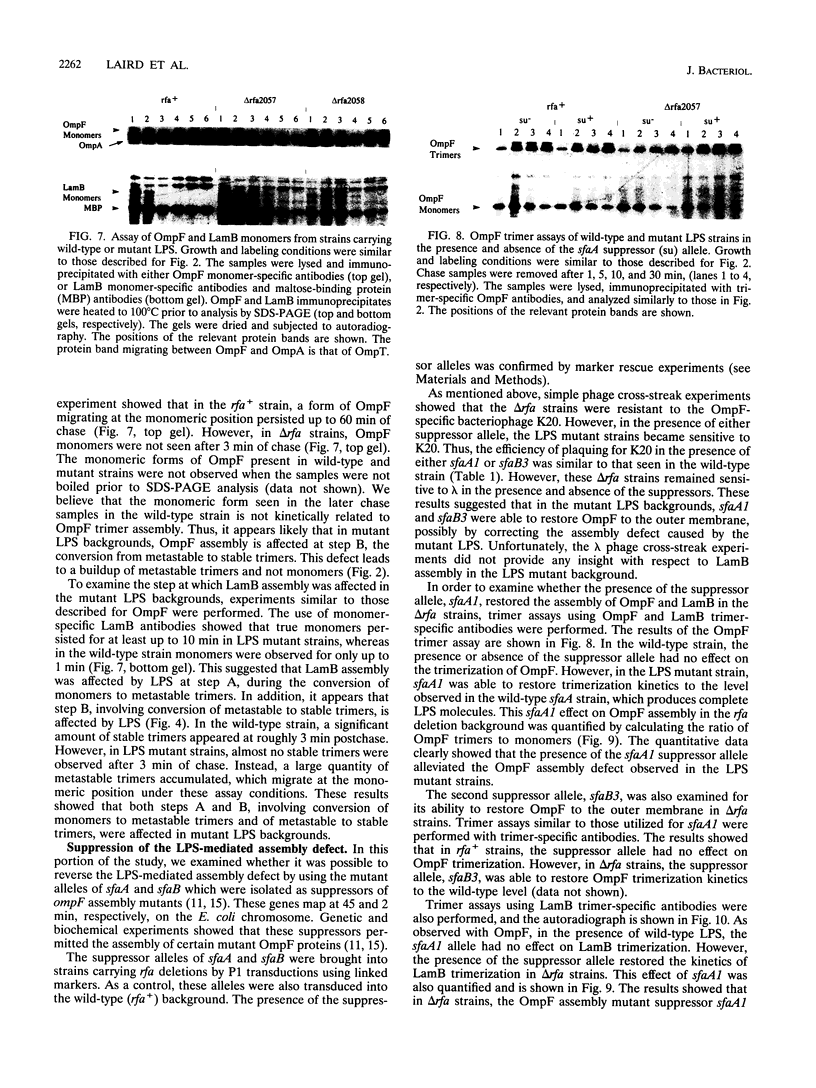
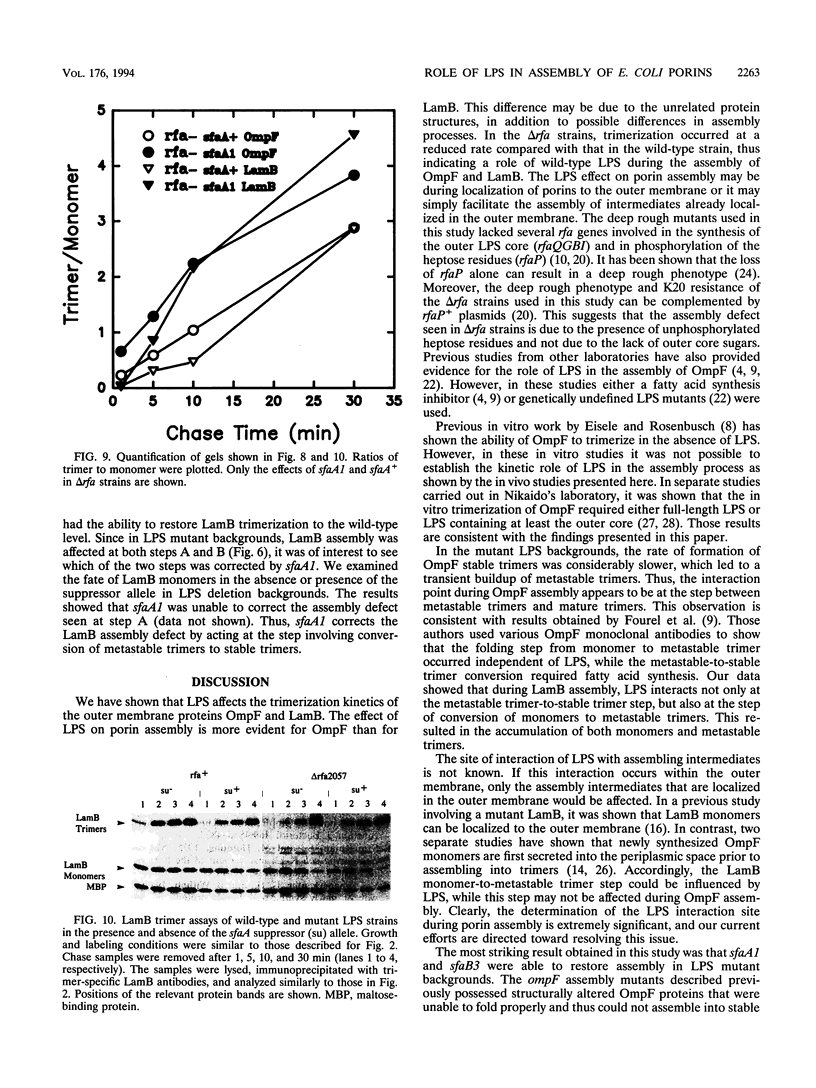
Assembly of the OmpF and LamB proteins was kinetically retarded in deep rough lipopolysaccharide mutants of Escherichia coli K-12. OmpF assembly was affected at the step of conversion of metastable trimers to stable trimers, whereas LamB assembly was influenced both at the monomer-to-metastable trimer and metastable-to-stable trimer steps. These assembly defects were reversed in the presence of the sfaA1 and sfaB3 suppressor alleles, which were isolated by using ompF assembly mutants.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames G. F., Spudich E. N., Nikaido H. Protein composition of the outer membrane of Salmonella typhimurium: effect of lipopolysaccharide mutations. J Bacteriol. 1974 Feb;117(2):406–416. doi: 10.1128/jb.117.2.406-416.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker K., Mackman N., Holland I. B. Genetics and biochemistry of the assembly of proteins into the outer membrane of E. coli. Prog Biophys Mol Biol. 1987;49(2-3):89–115. doi: 10.1016/0079-6107(87)90010-1. [DOI] [PubMed] [Google Scholar]
- Benz R. Structure and function of porins from gram-negative bacteria. Annu Rev Microbiol. 1988;42:359–393. doi: 10.1146/annurev.mi.42.100188.002043. [DOI] [PubMed] [Google Scholar]
- Bolla J. M., Lazdunski C., Pagès J. M. The assembly of the major outer membrane protein OmpF of Escherichia coli depends on lipid synthesis. EMBO J. 1988 Nov;7(11):3595–3599. doi: 10.1002/j.1460-2075.1988.tb03237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadaban M. J. Transposition and fusion of the lac genes to selected promoters in Escherichia coli using bacteriophage lambda and Mu. J Mol Biol. 1976 Jul 5;104(3):541–555. doi: 10.1016/0022-2836(76)90119-4. [DOI] [PubMed] [Google Scholar]
- Clément J. M., Hofnung M. Gene sequence of the lambda receptor, an outer membrane protein of E. coli K12. Cell. 1981 Dec;27(3 Pt 2):507–514. doi: 10.1016/0092-8674(81)90392-5. [DOI] [PubMed] [Google Scholar]
- Diedrich D. L., Stein M. A., Schnaitman C. A. Associations of Escherichia coli K-12 OmpF trimers with rough and smooth lipopolysaccharides. J Bacteriol. 1990 Sep;172(9):5307–5311. doi: 10.1128/jb.172.9.5307-5311.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisele J. L., Rosenbusch J. P. In vitro folding and oligomerization of a membrane protein. Transition of bacterial porin from random coil to native conformation. J Biol Chem. 1990 Jun 25;265(18):10217–10220. [PubMed] [Google Scholar]
- Fourel D., Mizushima S., Pagès J. M. Dynamics of the exposure of epitopes on OmpF, an outer membrane protein of Escherichia coli. Eur J Biochem. 1992 May 15;206(1):109–114. doi: 10.1111/j.1432-1033.1992.tb16907.x. [DOI] [PubMed] [Google Scholar]
- Helander I. M., Vaara M., Sukupolvi S., Rhen M., Saarela S., Zähringer U., Mäkelä P. H. rfaP mutants of Salmonella typhimurium. Eur J Biochem. 1989 Nov 20;185(3):541–546. doi: 10.1111/j.1432-1033.1989.tb15147.x. [DOI] [PubMed] [Google Scholar]
- Koplow J., Goldfine H. Alterations in the outer membrane of the cell envelope of heptose-deficient mutants of Escherichia coli. J Bacteriol. 1974 Feb;117(2):527–543. doi: 10.1128/jb.117.2.527-543.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugtenberg B., Meijers J., Peters R., van der Hoek P., van Alphen L. Electrophoretic resolution of the "major outer membrane protein" of Escherichia coli K12 into four bands. FEBS Lett. 1975 Oct 15;58(1):254–258. doi: 10.1016/0014-5793(75)80272-9. [DOI] [PubMed] [Google Scholar]
- Misra R. OmpF assembly mutants of Escherichia coli K-12: isolation, characterization, and suppressor analysis. J Bacteriol. 1993 Aug;175(16):5049–5056. doi: 10.1128/jb.175.16.5049-5056.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra R., Peterson A., Ferenci T., Silhavy T. J. A genetic approach for analyzing the pathway of LamB assembly into the outer membrane of Escherichia coli. J Biol Chem. 1991 Jul 25;266(21):13592–13597. [PubMed] [Google Scholar]
- Mühlradt P. F., Golecki J. R. Asymmetrical distribution and artifactual reorientation of lipopolysaccharide in the outer membrane bilayer of Salmonella typhimurium. Eur J Biochem. 1975 Feb 21;51(2):343–352. doi: 10.1111/j.1432-1033.1975.tb03934.x. [DOI] [PubMed] [Google Scholar]
- Nakae T. Outer membrane of Salmonella. Isolation of protein complex that produces transmembrane channels. J Biol Chem. 1976 Apr 10;251(7):2176–2178. [PubMed] [Google Scholar]
- Nikaido H., Vaara M. Molecular basis of bacterial outer membrane permeability. Microbiol Rev. 1985 Mar;49(1):1–32. doi: 10.1128/mr.49.1.1-32.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker C. T., Kloser A. W., Schnaitman C. A., Stein M. A., Gottesman S., Gibson B. W. Role of the rfaG and rfaP genes in determining the lipopolysaccharide core structure and cell surface properties of Escherichia coli K-12. J Bacteriol. 1992 Apr;174(8):2525–2538. doi: 10.1128/jb.174.8.2525-2538.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall-Hazelbauer L., Schwartz M. Isolation of the bacteriophage lambda receptor from Escherichia coli. J Bacteriol. 1973 Dec;116(3):1436–1446. doi: 10.1128/jb.116.3.1436-1446.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid J., Fung H., Gehring K., Klebba P. E., Nikaido H. Targeting of porin to the outer membrane of Escherichia coli. Rate of trimer assembly and identification of a dimer intermediate. J Biol Chem. 1988 Jun 5;263(16):7753–7759. [PubMed] [Google Scholar]
- Ried G., Hindennach I., Henning U. Role of lipopolysaccharide in assembly of Escherichia coli outer membrane proteins OmpA, OmpC, and OmpF. J Bacteriol. 1990 Oct;172(10):6048–6053. doi: 10.1128/jb.172.10.6048-6053.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaitman C. A., Klena J. D. Genetics of lipopolysaccharide biosynthesis in enteric bacteria. Microbiol Rev. 1993 Sep;57(3):655–682. doi: 10.1128/mr.57.3.655-682.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen K., Nikaido H. In vitro trimerization of OmpF porin secreted by spheroplasts of Escherichia coli. Proc Natl Acad Sci U S A. 1990 Jan;87(2):743–747. doi: 10.1073/pnas.87.2.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen K., Nikaido H. Lipopolysaccharide structure required for in vitro trimerization of Escherichia coli OmpF porin. J Bacteriol. 1991 Jan;173(2):926–928. doi: 10.1128/jb.173.2.926-928.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen K., Nikaido H. Trimerization of an in vitro synthesized OmpF porin of Escherichia coli outer membrane. J Biol Chem. 1991 Jun 15;266(17):11295–11300. [PubMed] [Google Scholar]
- Silverman J. A., Benson S. A. Bacteriophage K20 requires both the OmpF porin and lipopolysaccharide for receptor function. J Bacteriol. 1987 Oct;169(10):4830–4833. doi: 10.1128/jb.169.10.4830-4833.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szmelcman S., Hofnung M. Maltose transport in Escherichia coli K-12: involvement of the bacteriophage lambda receptor. J Bacteriol. 1975 Oct;124(1):112–118. doi: 10.1128/jb.124.1.112-118.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos-Scheperkeuter G. H., Witholt B. Assembly pathway of newly synthesized LamB protein an outer membrane protein of Escherichia coli K-12. J Mol Biol. 1984 Jun 5;175(4):511–528. doi: 10.1016/0022-2836(84)90182-7. [DOI] [PubMed] [Google Scholar]









