Abstract
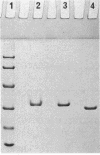
For the first time, a halogenating enzyme which is not known to produce halogenated metabolites has been isolated from a bacterial strain. The gene encoding the nonheme chloroperoxidase (CPO-L) from Streptomyces lividans TK64 was cloned, and its gene product was characterized. S. lividans TK64 produced only very small amounts of the enzyme. After cloning of the gene into Streptomyces aureofaciens Tü24-88, the enzyme was overexpressed up to 3,000-fold. Based on the overexpression, a simple purification procedure using acid precipitation and hydrophobic interaction chromatography was developed. Thus, 54 mg of homogeneous CPO-L could be obtained from 27 g (wet weight) of mycelium. The native enzyme has a molecular weight of 64,000 and consists of two identical subunits. The enzyme does not exhibit an absorption peak in the Soret region of the optical spectrum. X-ray fluorescence spectroscopy revealed that the enzyme does not contain any metal ions in equimolar amounts. CPO-L showed cross-reaction with antibodies raised against the nonheme chloroperoxidase from Pseudomonas pyrrocinia but not with antibodies raised against CPO-T from S. aureofaciens Tü24. CPO-L exhibits substrate specificity only for chlorination, not for bromination. Therefore, monochlorodimedone is only brominated by CPO-L, whereas indole is brominated and chlorinated. The functional chloroperoxidase gene was located on a 1.9-kb SalI DNA fragment. DNA sequence analysis revealed an open reading frame encoding a predicted polypeptide of 276 amino acids. The overall identity of the amino acid sequence to that of chloroperoxidase from P. pyrrocinia was 71%, whereas that to bromoperoxidase BPO-A2 from S. aureofaciens ATCC 10762 was only 42%.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akhrem A. A., Drozhdenyuk A. P. Calcium tartrate gel. Anal Biochem. 1989 May 15;179(1):86–89. doi: 10.1016/0003-2697(89)90205-4. [DOI] [PubMed] [Google Scholar]
- Altschul S. F., Gish W., Miller W., Myers E. W., Lipman D. J. Basic local alignment search tool. J Mol Biol. 1990 Oct 5;215(3):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich J., Bartz Q. R., Smith R. M., Joslyn D. A., Burkholder P. R. Chloromycetin, a New Antibiotic From a Soil Actinomycete. Science. 1947 Oct 31;106(2757):417–417. doi: 10.1126/science.106.2757.417. [DOI] [PubMed] [Google Scholar]
- Gribskov M., Devereux J., Burgess R. R. The codon preference plot: graphic analysis of protein coding sequences and prediction of gene expression. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 2):539–549. doi: 10.1093/nar/12.1part2.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hager L. P., Morris D. R., Brown F. S., Eberwein H. Chloroperoxidase. II. Utilization of halogen anions. J Biol Chem. 1966 Apr 25;241(8):1769–1777. [PubMed] [Google Scholar]
- Hopwood D. A., Kieser T., Wright H. M., Bibb M. J. Plasmids, recombination and chromosome mapping in Streptomyces lividans 66. J Gen Microbiol. 1983 Jul;129(7):2257–2269. doi: 10.1099/00221287-129-7-2257. [DOI] [PubMed] [Google Scholar]
- Kieser T. Factors affecting the isolation of CCC DNA from Streptomyces lividans and Escherichia coli. Plasmid. 1984 Jul;12(1):19–36. doi: 10.1016/0147-619x(84)90063-5. [DOI] [PubMed] [Google Scholar]
- Knoch M., van Pée K. H., Vining L. C., Lingens F. Purification, properties and immunological detection of a bromoperoxidase-catalase from Streptomyces venezuelae and from a chloramphenicol-nonproducing mutant. J Gen Microbiol. 1989 Sep;135(9):2493–2502. doi: 10.1099/00221287-135-9-2493. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mandel M., Higa A. Calcium-dependent bacteriophage DNA infection. J Mol Biol. 1970 Oct 14;53(1):159–162. doi: 10.1016/0022-2836(70)90051-3. [DOI] [PubMed] [Google Scholar]
- Morris D. R., Hager L. P. Chloroperoxidase. I. Isolation and properties of the crystalline glycoprotein. J Biol Chem. 1966 Apr 25;241(8):1763–1768. [PubMed] [Google Scholar]
- Olsen R. L., Little C. Purification and some properties of myeloperoxidase and eosinophil peroxidase from human blood. Biochem J. 1983 Mar 1;209(3):781–787. doi: 10.1042/bj2090781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer O., Pelletier I., Altenbuchner J., van Pée K. H. Molecular cloning and sequencing of a non-haem bromoperoxidase gene from Streptomyces aureofaciens ATCC 10762. J Gen Microbiol. 1992 Jun;138(6):1123–1131. doi: 10.1099/00221287-138-6-1123. [DOI] [PubMed] [Google Scholar]
- Schägger H., von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987 Nov 1;166(2):368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- Strohl W. R. Compilation and analysis of DNA sequences associated with apparent streptomycete promoters. Nucleic Acids Res. 1992 Mar 11;20(5):961–974. doi: 10.1093/nar/20.5.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward J. M., Janssen G. R., Kieser T., Bibb M. J., Buttner M. J., Bibb M. J. Construction and characterisation of a series of multi-copy promoter-probe plasmid vectors for Streptomyces using the aminoglycoside phosphotransferase gene from Tn5 as indicator. Mol Gen Genet. 1986 Jun;203(3):468–478. doi: 10.1007/BF00422072. [DOI] [PubMed] [Google Scholar]
- Weng M., Pfeifer O., Krauss S., Lingens F., van Pée K. H. Purification, characterization and comparison of two non-haem bromoperoxidases from Streptomyces aureofaciens ATCC 10762. J Gen Microbiol. 1991 Nov;137(11):2539–2546. doi: 10.1099/00221287-137-11-2539. [DOI] [PubMed] [Google Scholar]
- Wiesner W., van Pee K. H., Lingens F. Detection of a new chloroperoxidase in Pseudomonas pyrrocinia. FEBS Lett. 1986 Dec 15;209(2):321–324. doi: 10.1016/0014-5793(86)81135-8. [DOI] [PubMed] [Google Scholar]
- Wiesner W., van Pée K. H., Lingens F. Purification and characterization of a novel bacterial non-heme chloroperoxidase from Pseudomonas pyrrocinia. J Biol Chem. 1988 Sep 25;263(27):13725–13732. [PubMed] [Google Scholar]
- Wolfframm C., Lingens F., Mutzel R., van Pée K. H. Chloroperoxidase-encoding gene from Pseudomonas pyrrocinia: sequence, expression in heterologous hosts, and purification of the enzyme. Gene. 1993 Aug 16;130(1):131–135. doi: 10.1016/0378-1119(93)90356-8. [DOI] [PubMed] [Google Scholar]
- Wolfframm C., van Pée K. H., Lingens F. Cloning and high-level expression of a chloroperoxidase gene from Pseudomonas pyrrocinia in Escherichia coli. FEBS Lett. 1988 Oct 10;238(2):325–328. doi: 10.1016/0014-5793(88)80505-2. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- van Pee K. H., Sury G., Lingens F. Purification and properties of a nonheme bromoperoxidase from Streptomyces aureofaciens. Biol Chem Hoppe Seyler. 1987 Sep;368(9):1225–1232. doi: 10.1515/bchm3.1987.368.2.1225. [DOI] [PubMed] [Google Scholar]
- van Pée K. H., Lingens F. Purification and molecular and catalytic properties of bromoperoxidase from Streptomyces phaeochromogenes. J Gen Microbiol. 1985 Aug;131(8):1911–1916. doi: 10.1099/00221287-131-8-1911. [DOI] [PubMed] [Google Scholar]
- van Pée K. H., Lingens F. Purification of bromoperoxidase from Pseudomonas aureofaciens. J Bacteriol. 1985 Mar;161(3):1171–1175. doi: 10.1128/jb.161.3.1171-1175.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Pée K. H. Molecular cloning and high-level expression of a bromoperoxidase gene from Streptomyces aureofaciens Tü24. J Bacteriol. 1988 Dec;170(12):5890–5894. doi: 10.1128/jb.170.12.5890-5894.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]