Abstract
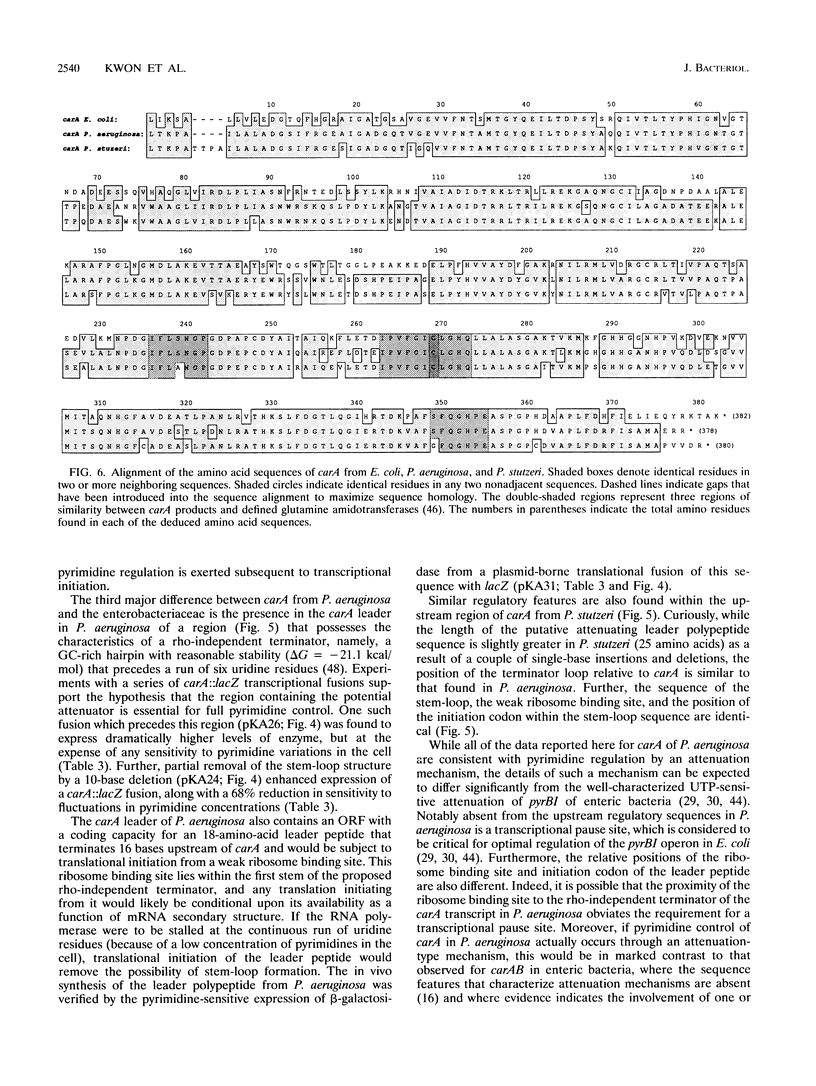
The carAB operons from Pseudomonas aeruginosa PAO1 and Pseudomonas stutzeri JM300 were characterized by Southern and DNA sequence analyses. The results show that the previously reported sequence for carA (S. C. Wong and A. T. Abdelal, J. Bacteriol. 172:630-642, 1990) is derived from P. stutzeri and not P. aeruginosa, as originally reported. Therefore, the amino-terminal sequence of the purified carA product is identical to that derived from the nucleotide sequence in both organisms, P. stutzeri having four additional amino acids. The results also show that while carA and carB are contiguous in P. stutzeri, as is the case in other bacteria, they are surprisingly separated by an open reading frame (ORF) of 216 amino acids in P. aeruginosa. S1 nuclease mapping experiments with RNA extracted under a variety of growth conditions, as well as experiments using different lacZ fusions, indicate that the carA-ORF-carB operon of P. aeruginosa is transcribed from a single promoter. Moreover, these experiments demonstrate that expression of this single transcript is controlled by both arginine and pyrimidines and that variation in arginine levels specifically modulates transcriptional initiation, while pyrimidine regulation is exerted subsequent to transcriptional initiation. Modification of a rho-independent terminator-like structure, which is present upstream of carA in P. aeruginosa, removed all transcriptional sensitivity of a carA::lacZ fusion to pyrimidines. This result, when coupled with the finding that translation of an 18-amino-acid leader polypeptide (associated with this putative rho-independent terminator), is inversely proportional to pyrimidine concentration in the cell, strongly suggests that regulation of carA by pyrimidines is mediated through an attenuation-type mechanism in P. aeruginosa.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdelal A. T., Bibb W. F., Nainan O. Carbamate kinase from Pseudomonas aeruginosa: purification, characterization, physiological role, and regulation. J Bacteriol. 1982 Sep;151(3):1411–1419. doi: 10.1128/jb.151.3.1411-1419.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdelal A. T., Bussey L., Vickers L. Carbamoylphosphate synthetase from Pseudomonas aeruginosa. Subunit composition, kinetic analysis and regulation. Eur J Biochem. 1983 Jan 1;129(3):697–702. [PubMed] [Google Scholar]
- Abdelal A. T., Ingraham J. L. Carbamylphosphate synthetase from Salmonella typhimurium. Regulations, subunit composition, and function of the subunits. J Biol Chem. 1975 Jun 25;250(12):4410–4417. [PubMed] [Google Scholar]
- Aldrich T. L., Frantz B., Gill J. F., Kilbane J. J., Chakrabarty A. M. Cloning and complete nucleotide sequence determination of the catB gene encoding cis,cis-muconate lactonizing enzyme. Gene. 1987;52(2-3):185–195. doi: 10.1016/0378-1119(87)90045-x. [DOI] [PubMed] [Google Scholar]
- Berry D., Kropinski A. M. Effect of lipopolysaccharide mutations and temperature on plasmid transformation efficiency in Pseudomonas aeruginosa. Can J Microbiol. 1986 May;32(5):436–438. doi: 10.1139/m86-082. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C. A rapid alkaline extraction method for the isolation of plasmid DNA. Methods Enzymol. 1983;100:243–255. doi: 10.1016/0076-6879(83)00059-2. [DOI] [PubMed] [Google Scholar]
- Bouvier J., Patte J. C., Stragier P. Multiple regulatory signals in the control region of the Escherichia coli carAB operon. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4139–4143. doi: 10.1073/pnas.81.13.4139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouvier J., Richaud C., Richaud F., Patte J. C., Stragier P. Nucleotide sequence and expression of the Escherichia coli dapB gene. J Biol Chem. 1984 Dec 10;259(23):14829–14834. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Carlson C. A., Pierson L. S., Rosen J. J., Ingraham J. L. Pseudomonas stutzeri and related species undergo natural transformation. J Bacteriol. 1983 Jan;153(1):93–99. doi: 10.1128/jb.153.1.93-99.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadaban M. J., Martinez-Arias A., Shapira S. K., Chou J. Beta-galactosidase gene fusions for analyzing gene expression in escherichia coli and yeast. Methods Enzymol. 1983;100:293–308. doi: 10.1016/0076-6879(83)00063-4. [DOI] [PubMed] [Google Scholar]
- Chang M., Crawford I. P. The roles of indoleglycerol phosphate and the TrpI protein in the expression of trpBA from Pseudomonas aeruginosa. Nucleic Acids Res. 1990 Feb 25;18(4):979–988. doi: 10.1093/nar/18.4.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlier D., Roovers M., Gigot D., Huysveld N., Piérard A., Glansdorff N. Integration host factor (IHF) modulates the expression of the pyrimidine-specific promoter of the carAB operons of Escherichia coli K12 and Salmonella typhimurium LT2. Mol Gen Genet. 1993 Feb;237(1-2):273–286. doi: 10.1007/BF00282809. [DOI] [PubMed] [Google Scholar]
- Charlier D., Roovers M., Van Vliet F., Boyen A., Cunin R., Nakamura Y., Glansdorff N., Piérard A. Arginine regulon of Escherichia coli K-12. A study of repressor-operator interactions and of in vitro binding affinities versus in vivo repression. J Mol Biol. 1992 Jul 20;226(2):367–386. doi: 10.1016/0022-2836(92)90953-h. [DOI] [PubMed] [Google Scholar]
- Crabeel M., Charlier D., Weyens G., Feller A., Piérard A., Glansdorff N. Use of gene cloning to determine polarity of an operon: genes carAB of Escherichia coli. J Bacteriol. 1980 Aug;143(2):921–925. doi: 10.1128/jb.143.2.921-925.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunin R., Glansdorff N., Piérard A., Stalon V. Biosynthesis and metabolism of arginine in bacteria. Microbiol Rev. 1986 Sep;50(3):314–352. doi: 10.1128/mr.50.3.314-352.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans G. A., Lewis K., Rothenberg B. E. High efficiency vectors for cosmid microcloning and genomic analysis. Gene. 1989 Jun 30;79(1):9–20. doi: 10.1016/0378-1119(89)90088-7. [DOI] [PubMed] [Google Scholar]
- Farinha M. A., Kropinski A. M. Construction of broad-host-range plasmid vectors for easy visible selection and analysis of promoters. J Bacteriol. 1990 Jun;172(6):3496–3499. doi: 10.1128/jb.172.6.3496-3499.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Gribskov M., Devereux J., Burgess R. R. The codon preference plot: graphic analysis of protein coding sequences and prediction of gene expression. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 2):539–549. doi: 10.1093/nar/12.1part2.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillou F., Rubino S. D., Markovitz R. S., Kinney D. M., Lusty C. J. Escherichia coli carbamoyl-phosphate synthetase: domains of glutaminase and synthetase subunit interaction. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8304–8308. doi: 10.1073/pnas.86.21.8304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas D., Holloway B. W., Schamböck A., Leisinger T. The genetic organization of arginine biosynthesis in Pseudomonas aeruginosa. Mol Gen Genet. 1977 Jul 7;154(1):7–22. doi: 10.1007/BF00265571. [DOI] [PubMed] [Google Scholar]
- Han B. D., Nolan W. G., Hopkins H. P., Jones R. T., Ingraham J. L., Abdelal A. T. Effect of growth temperature on folding of carbamoylphosphate synthetases of Salmonella typhimurium and a cold-sensitive derivative. J Bacteriol. 1990 Sep;172(9):5089–5096. doi: 10.1128/jb.172.9.5089-5096.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C. G., Turnbough C. L., Jr Multiple control mechanisms for pyrimidine-mediated regulation of pyrBI operon expression in Escherichia coli K-12. J Bacteriol. 1989 Jun;171(6):3337–3342. doi: 10.1128/jb.171.6.3337-3342.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu C. D., Abdelal A. T. The Salmonella typhimurium uracil-sensitive mutation use is in argU and encodes a minor arginine tRNA. J Bacteriol. 1993 Jun;175(12):3897–3899. doi: 10.1128/jb.175.12.3897-3899.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu C. D., Houghton J. E., Abdelal A. T. Characterization of the arginine repressor from Salmonella typhimurium and its interactions with the carAB operator. J Mol Biol. 1992 May 5;225(1):11–24. doi: 10.1016/0022-2836(92)91022-h. [DOI] [PubMed] [Google Scholar]
- Lu C. D., Kilstrup M., Neuhard J., Abdelal A. Pyrimidine regulation of tandem promoters for carAB in Salmonella typhimurium. J Bacteriol. 1989 Oct;171(10):5436–5442. doi: 10.1128/jb.171.10.5436-5442.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem. 1987 Jul 25;262(21):10035–10038. [PubMed] [Google Scholar]
- Mierendorf R. C., Pfeffer D. Direct sequencing of denatured plasmid DNA. Methods Enzymol. 1987;152:556–562. doi: 10.1016/0076-6879(87)52061-4. [DOI] [PubMed] [Google Scholar]
- Piette J., Nyunoya H., Lusty C. J., Cunin R., Weyens G., Crabeel M., Charlier D., Glansdorff N., Piérard A. DNA sequence of the carA gene and the control region of carAB: tandem promoters, respectively controlled by arginine and the pyrimidines, regulate the synthesis of carbamoyl-phosphate synthetase in Escherichia coli K-12. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4134–4138. doi: 10.1073/pnas.81.13.4134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn C. L., Stephenson B. T., Switzer R. L. Functional organization and nucleotide sequence of the Bacillus subtilis pyrimidine biosynthetic operon. J Biol Chem. 1991 May 15;266(14):9113–9127. [PubMed] [Google Scholar]
- Ratnaningsih E., Dharmsthiti S., Krishnapillai V., Morgan A., Sinclair M., Holloway B. W. A combined physical and genetic map of Pseudomonas aeruginosa PAO. J Gen Microbiol. 1990 Dec;136(12):2351–2357. doi: 10.1099/00221287-136-12-2351. [DOI] [PubMed] [Google Scholar]
- Roland K. L., Powell F. E., Turnbough C. L., Jr Role of translation and attenuation in the control of pyrBI operon expression in Escherichia coli K-12. J Bacteriol. 1985 Sep;163(3):991–999. doi: 10.1128/jb.163.3.991-999.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stalon V., Ramos F., Piérard A., Wiame J. M. The occurrence of a catabolic and an anabolic ornithine carbamoyltransferase in Pseudomonas. Biochim Biophys Acta. 1967 May 16;139(1):91–97. doi: 10.1016/0005-2744(67)90115-5. [DOI] [PubMed] [Google Scholar]
- Tian G., Lim D., Carey J., Maas W. K. Binding of the arginine repressor of Escherichia coli K12 to its operator sites. J Mol Biol. 1992 Jul 20;226(2):387–397. doi: 10.1016/0022-2836(92)90954-i. [DOI] [PubMed] [Google Scholar]
- Turnbough C. L., Jr, Hicks K. L., Donahue J. P. Attenuation control of pyrBI operon expression in Escherichia coli K-12. Proc Natl Acad Sci U S A. 1983 Jan;80(2):368–372. doi: 10.1073/pnas.80.2.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J., Messing J. Production of single-stranded plasmid DNA. Methods Enzymol. 1987;153:3–11. doi: 10.1016/0076-6879(87)53044-0. [DOI] [PubMed] [Google Scholar]
- Werner M., Feller A., Piérard A. Nucleotide sequence of yeast gene CP A1 encoding the small subunit of arginine-pathway carbamoyl-phosphate synthetase. Homology of the deduced amino acid sequence to other glutamine amidotransferases. Eur J Biochem. 1985 Jan 15;146(2):371–381. doi: 10.1111/j.1432-1033.1985.tb08663.x. [DOI] [PubMed] [Google Scholar]
- Wong S. C., Abdelal A. T. Unorthodox expression of an enzyme: evidence for an untranslated region within carA from Pseudomonas aeruginosa. J Bacteriol. 1990 Feb;172(2):630–642. doi: 10.1128/jb.172.2.630-642.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]