Abstract
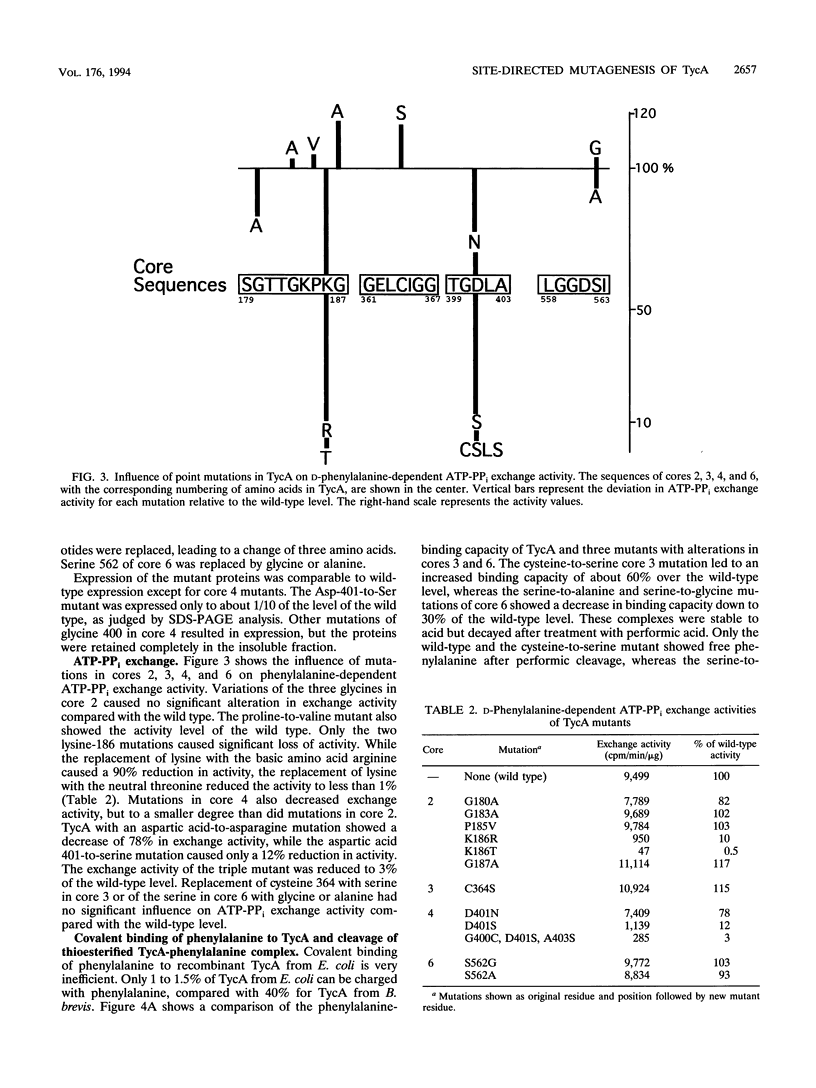
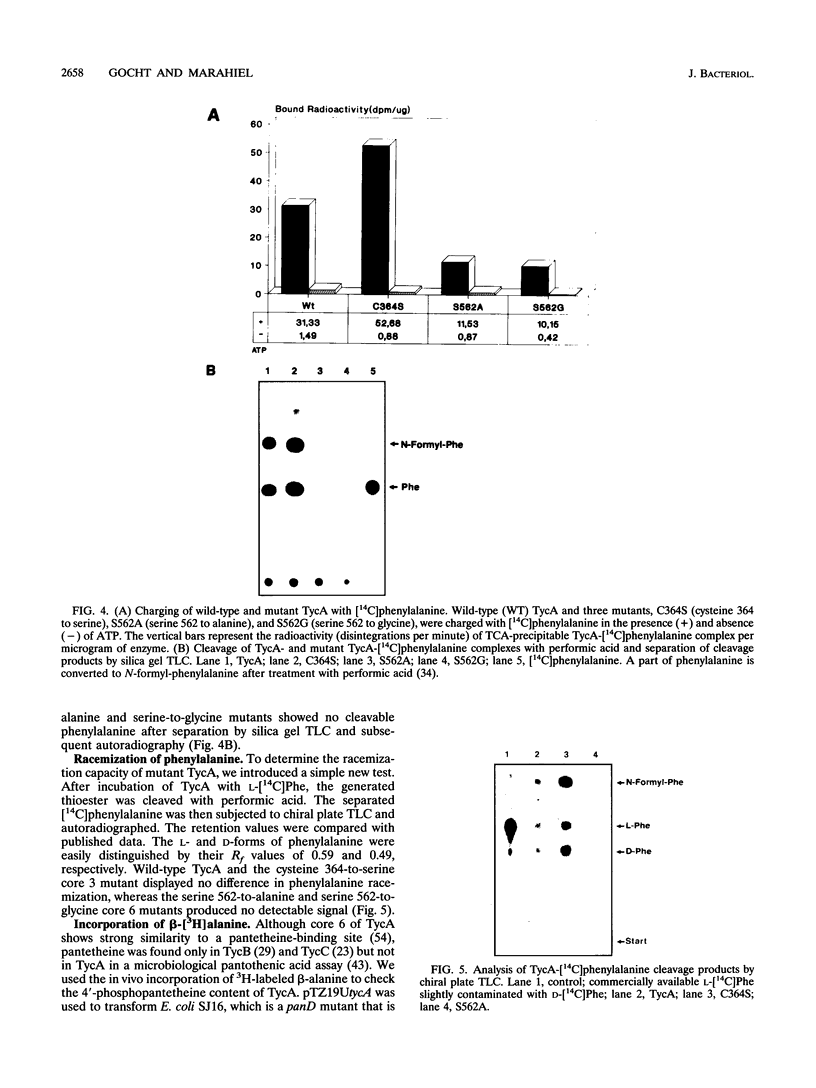
The D-phenylalanine-activating enzyme tyrocidine synthetase I (TycA) from Bacillus brevis ATCC 8185 was overexpressed in Escherichia coli, purified to homogeneity, and assayed for ATP-PPi exchange and covalent binding of phenylalanine by the thiotemplate mechanism. Amino acid exchanges in four different cores of TycA created by site-directed mutagenesis revealed the amino acid residues involved in aminoacyladenylate formation and in covalent thioester formation. Mutations in the putative ATP-binding site SGTTGKPKG caused a decreased phenylalanine-dependent ATP-PPi exchange activity to 10% of the wild-type level for a Lys-186-to-Arg substitution and an almost complete loss of activity (< 1%) for a Lys-186-to-Thr exchange. Alteration of Asp-401 to Asn in the ATPase motif TGDL of TycA decreased the phenylalanine-dependent ATP-PPi exchange activity to 75% of wild type, while an Asp-401-to-Ser mutation decreased the activity to 10% of the wild-type level. Replacement of Ser-562 in the putative thioester-binding motif LGGDSI to Ala or Gly caused a reduction in trichloroacetic acid-precipitable TycA-[14C]phenylalanine complex to one-third of the wild-type level. However, no cleavable [14C]phenylalanine could be detected after treatment with performic acid, indicating that the resulting mutant was unable to form thioester with phenylalanine. In E. coli, TycA was labeled with beta-[3H]alanine, a precursor of 4'-phosphopantetheine, indicating that TycA is modified with a beta-alanine-containing cofactor.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Addison R. Primary structure of the Neurospora plasma membrane H+-ATPase deduced from the gene sequence. Homology to Na+/K+-, Ca2+-, and K+-ATPase. J Biol Chem. 1986 Nov 15;261(32):14896–14901. [PubMed] [Google Scholar]
- Akers H. A., Lee S. G., Lipmann F. Identification of two enzymes responsible for the synthesis of the initial portion of linear gramicidin. Biochemistry. 1977 Dec 27;16(26):5722–5729. doi: 10.1021/bi00645a012. [DOI] [PubMed] [Google Scholar]
- Alberts A. W., Vagelos P. R. Acyl carrier protein. 8. Studies of acyl carrier protein and coenzyme A in Escherichia coli pantothenate or betaalanine auxotrophs. J Biol Chem. 1966 Nov 25;241(22):5201–5204. [PubMed] [Google Scholar]
- Bordo D., Argos P. Suggestions for "safe" residue substitutions in site-directed mutagenesis. J Mol Biol. 1991 Feb 20;217(4):721–729. doi: 10.1016/0022-2836(91)90528-e. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cosmina P., Rodriguez F., de Ferra F., Grandi G., Perego M., Venema G., van Sinderen D. Sequence and analysis of the genetic locus responsible for surfactin synthesis in Bacillus subtilis. Mol Microbiol. 1993 May;8(5):821–831. doi: 10.1111/j.1365-2958.1993.tb01629.x. [DOI] [PubMed] [Google Scholar]
- Cronan J. E., Jr Beta-alanine synthesis in Escherichia coli. J Bacteriol. 1980 Mar;141(3):1291–1297. doi: 10.1128/jb.141.3.1291-1297.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Souza C., Nakano M. M., Corbell N., Zuber P. Amino-acylation site mutations in amino acid-activating domains of surfactin synthetase: effects on surfactin production and competence development in Bacillus subtilis. J Bacteriol. 1993 Jun;175(11):3502–3510. doi: 10.1128/jb.175.11.3502-3510.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis C. B., Smith K. E., Campbell B. N., Jr, Hammes G. G. The ATP binding site of the yeast plasma membrane proton-translocating ATPase. J Biol Chem. 1990 Jan 25;265(3):1300–1305. [PubMed] [Google Scholar]
- Fuma S., Fujishima Y., Corbell N., D'Souza C., Nakano M. M., Zuber P., Yamane K. Nucleotide sequence of 5' portion of srfA that contains the region required for competence establishment in Bacillus subtilus. Nucleic Acids Res. 1993 Jan 11;21(1):93–97. doi: 10.1093/nar/21.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fürbass R., Gocht M., Zuber P., Marahiel M. A. Interaction of AbrB, a transcriptional regulator from Bacillus subtilis with the promoters of the transition state-activated genes tycA and spoVG. Mol Gen Genet. 1991 Mar;225(3):347–354. doi: 10.1007/BF00261673. [DOI] [PubMed] [Google Scholar]
- Geiger O., Spaink H. P., Kennedy E. P. Isolation of the Rhizobium leguminosarum NodF nodulation protein: NodF carries a 4'-phosphopantetheine prosthetic group. J Bacteriol. 1991 May;173(9):2872–2878. doi: 10.1128/jb.173.9.2872-2878.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerra D. J., Dziewanowska K., Ohlrogge J. B., Beremand P. D. Purification and characterization of recombinant spinach acyl carrier protein I expressed in Escherichia coli. J Biol Chem. 1988 Mar 25;263(9):4386–4391. [PubMed] [Google Scholar]
- Higgins C. F., Hiles I. D., Salmond G. P., Gill D. R., Downie J. A., Evans I. J., Holland I. B., Gray L., Buckel S. D., Bell A. W. A family of related ATP-binding subunits coupled to many distinct biological processes in bacteria. Nature. 1986 Oct 2;323(6087):448–450. doi: 10.1038/323448a0. [DOI] [PubMed] [Google Scholar]
- Hori K., Yamamoto Y., Minetoki T., Kurotsu T., Kanda M., Miura S., Okamura K., Furuyama J., Saito Y. Molecular cloning and nucleotide sequence of the gramicidin S synthetase 1 gene. J Biochem. 1989 Oct;106(4):639–645. doi: 10.1093/oxfordjournals.jbchem.a122909. [DOI] [PubMed] [Google Scholar]
- Jackowski S., Rock C. O. Ratio of active to inactive forms of acyl carrier protein in Escherichia coli. J Biol Chem. 1983 Dec 25;258(24):15186–15191. [PubMed] [Google Scholar]
- Jackowski S., Rock C. O. Regulation of coenzyme A biosynthesis. J Bacteriol. 1981 Dec;148(3):926–932. doi: 10.1128/jb.148.3.926-932.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanda M., Hori K., Kurotsu T., Miura S., Saito Y. Reaction mechanism of gramicidin S synthetase 1, phenylalanine racemase, of Bacillus brevis. J Biochem. 1989 Apr;105(4):653–659. doi: 10.1093/oxfordjournals.jbchem.a122720. [DOI] [PubMed] [Google Scholar]
- Kleinkauf H., Gevers W., Roskoski R., Jr, Lipmann F. Enzyme-bound phosphopantetheine in tyrocidine biosynthesis. Biochem Biophys Res Commun. 1970 Dec 9;41(5):1218–1222. doi: 10.1016/0006-291x(70)90216-0. [DOI] [PubMed] [Google Scholar]
- Kleinkauf H., von Döhren H. Nonribosomal biosynthesis of peptide antibiotics. Eur J Biochem. 1990 Aug 28;192(1):1–15. doi: 10.1111/j.1432-1033.1990.tb19188.x. [DOI] [PubMed] [Google Scholar]
- Klose M., Schimz K. L., van der Wolk J., Driessen A. J., Freudl R. Lysine 106 of the putative catalytic ATP-binding site of the Bacillus subtilis SecA protein is required for functional complementation of Escherichia coli secA mutants in vivo. J Biol Chem. 1993 Feb 25;268(6):4504–4510. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee S. G., Lipmann F. Isolation of a peptidyl-pantetheine-protein from tyrocidine-synthesizing polyenzymes. Proc Natl Acad Sci U S A. 1974 Mar;71(3):607–611. doi: 10.1073/pnas.71.3.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. G., Lipmann F. Isolation of amino acid activating subunit--pantetheine protein complexes: their role in chain elongation in tyrocidine synthesis. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2343–2347. doi: 10.1073/pnas.74.6.2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. G., Lipmann F. Tyrocidine synthetase system. Methods Enzymol. 1975;43:585–602. doi: 10.1016/0076-6879(75)43121-4. [DOI] [PubMed] [Google Scholar]
- Lee S. G., Roskoski R., Jr, Bauer K., Lipmann F. Purification of the polyenzymes responsible for tyrocidine synthesis and their dissociation into subunits. Biochemistry. 1973 Jan 30;12(3):398–405. doi: 10.1021/bi00727a006. [DOI] [PubMed] [Google Scholar]
- Lipmann F. Bacterial production of antibiotic polypeptides by thiol-linked synthesis on protein templates. Adv Microb Physiol. 1980;21:227–266. doi: 10.1016/s0065-2911(08)60357-4. [DOI] [PubMed] [Google Scholar]
- Lipmann F., Gevers W., Kleinkauf H., Roskoski R., Jr Polypeptide synthesis on protein templates: the enzymatic synthesis of gramicidin S and tyrocidine. Adv Enzymol Relat Areas Mol Biol. 1971;35:1–34. doi: 10.1002/9780470122808.ch1. [DOI] [PubMed] [Google Scholar]
- Lozoya E., Hoffmann H., Douglas C., Schulz W., Scheel D., Hahlbrock K. Primary structures and catalytic properties of isoenzymes encoded by the two 4-coumarate: CoA ligase genes in parsley. Eur J Biochem. 1988 Oct 1;176(3):661–667. doi: 10.1111/j.1432-1033.1988.tb14328.x. [DOI] [PubMed] [Google Scholar]
- Marahiel M. A., Krause M., Skarpeid H. J. Cloning of the tyrocidine synthetase 1 gene from Bacillus brevis and its expression in Escherichia coli. Mol Gen Genet. 1985;201(2):231–236. doi: 10.1007/BF00425664. [DOI] [PubMed] [Google Scholar]
- Marahiel M. A. Multidomain enzymes involved in peptide synthesis. FEBS Lett. 1992 Jul 27;307(1):40–43. doi: 10.1016/0014-5793(92)80898-q. [DOI] [PubMed] [Google Scholar]
- Marahiel M. A., Nakano M. M., Zuber P. Regulation of peptide antibiotic production in Bacillus. Mol Microbiol. 1993 Mar;7(5):631–636. doi: 10.1111/j.1365-2958.1993.tb01154.x. [DOI] [PubMed] [Google Scholar]
- Marahiel M. A., Zuber P., Czekay G., Losick R. Identification of the promoter for a peptide antibiotic biosynthesis gene from Bacillus brevis and its regulation in Bacillus subtilis. J Bacteriol. 1987 May;169(5):2215–2222. doi: 10.1128/jb.169.5.2215-2222.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinus M. G., Morris N. R. Isolation of deoxyribonucleic acid methylase mutants of Escherichia coli K-12. J Bacteriol. 1973 Jun;114(3):1143–1150. doi: 10.1128/jb.114.3.1143-1150.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Mittenhuber G., Weckermann R., Marahiel M. A. Gene cluster containing the genes for tyrocidine synthetases 1 and 2 from Bacillus brevis: evidence for an operon. J Bacteriol. 1989 Sep;171(9):4881–4887. doi: 10.1128/jb.171.9.4881-4887.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo J. P., Slayman C. W. The fluorescein isothiocyanate-binding site of the plasma-membrane H+-ATPase of Neurospora crassa. J Biol Chem. 1988 Dec 15;263(35):18664–18668. [PubMed] [Google Scholar]
- Portillo F., Serrano R. Dissection of functional domains of the yeast proton-pumping ATPase by directed mutagenesis. EMBO J. 1988 Jun;7(6):1793–1798. doi: 10.1002/j.1460-2075.1988.tb03010.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott D. J., Vagelos P. R. Acyl carrier protein. Adv Enzymol Relat Areas Mol Biol. 1972;36:269–311. doi: 10.1002/9780470122815.ch8. [DOI] [PubMed] [Google Scholar]
- Pugh E. L., Wakil S. J. Studies on the mechanism of fatty acid synthesis. XIV. The prosthetic group of acyl carrier protein and the mode of its attachment to the protein. J Biol Chem. 1965 Dec;240(12):4727–4733. [PubMed] [Google Scholar]
- Revill W. P., Leadlay P. F. Cloning, characterization, and high-level expression in Escherichia coli of the Saccharopolyspora erythraea gene encoding an acyl carrier protein potentially involved in fatty acid biosynthesis. J Bacteriol. 1991 Jul;173(14):4379–4385. doi: 10.1128/jb.173.14.4379-4385.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts G. A., Staunton J., Leadlay P. F. Heterologous expression in Escherichia coli of an intact multienzyme component of the erythromycin-producing polyketide synthase. Eur J Biochem. 1993 May 15;214(1):305–311. doi: 10.1111/j.1432-1033.1993.tb17925.x. [DOI] [PubMed] [Google Scholar]
- Robertson J. B., Gocht M., Marahiel M. A., Zuber P. AbrB, a regulator of gene expression in Bacillus, interacts with the transcription initiation regions of a sporulation gene and an antibiotic biosynthesis gene. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8457–8461. doi: 10.1073/pnas.86.21.8457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock C. O., Cronan J. E., Jr Acyl carrier protein from Escherichia coli. Methods Enzymol. 1981;71(Pt 100):341–351. doi: 10.1016/0076-6879(81)71043-7. [DOI] [PubMed] [Google Scholar]
- Rock C. O. Mixed disulfides of acyl carrier protein and coenzyme A with specific soluble proteins in Escherichia coli. J Bacteriol. 1982 Dec;152(3):1298–1300. doi: 10.1128/jb.152.3.1298-1300.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roskoski R., Jr, Kleinkauf H., Gevers W., Lipmann F. Isolation of enzyme-bound peptide intermediates in tyrocidine biosynthesis. Biochemistry. 1970 Dec 8;9(25):4846–4851. doi: 10.1021/bi00827a003. [DOI] [PubMed] [Google Scholar]
- Rusnak F., Sakaitani M., Drueckhammer D., Reichert J., Walsh C. T. Biosynthesis of the Escherichia coli siderophore enterobactin: sequence of the entF gene, expression and purification of EntF, and analysis of covalent phosphopantetheine. Biochemistry. 1991 Mar 19;30(11):2916–2927. doi: 10.1021/bi00225a027. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraste M., Sibbald P. R., Wittinghofer A. The P-loop--a common motif in ATP- and GTP-binding proteins. Trends Biochem Sci. 1990 Nov;15(11):430–434. doi: 10.1016/0968-0004(90)90281-f. [DOI] [PubMed] [Google Scholar]
- Schlumbohm W., Stein T., Ullrich C., Vater J., Krause M., Marahiel M. A., Kruft V., Wittmann-Liebold B. An active serine is involved in covalent substrate amino acid binding at each reaction center of gramicidin S synthetase. J Biol Chem. 1991 Dec 5;266(34):23135–23141. [PubMed] [Google Scholar]
- Serrano R., Kielland-Brandt M. C., Fink G. R. Yeast plasma membrane ATPase is essential for growth and has homology with (Na+ + K+), K+- and Ca2+-ATPases. Nature. 1986 Feb 20;319(6055):689–693. doi: 10.1038/319689a0. [DOI] [PubMed] [Google Scholar]
- Shen B., Summers R. G., Gramajo H., Bibb M. J., Hutchinson C. R. Purification and characterization of the acyl carrier protein of the Streptomyces glaucescens tetracenomycin C polyketide synthase. J Bacteriol. 1992 Jun;174(11):3818–3821. doi: 10.1128/jb.174.11.3818-3821.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J. W., Ott J., Eckstein F. The rapid generation of oligonucleotide-directed mutations at high frequency using phosphorothioate-modified DNA. Nucleic Acids Res. 1985 Dec 20;13(24):8765–8785. doi: 10.1093/nar/13.24.8765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turgay K., Krause M., Marahiel M. A. Four homologous domains in the primary structure of GrsB are related to domains in a superfamily of adenylate-forming enzymes. Mol Microbiol. 1992 Feb;6(4):529–546. doi: 10.1111/j.1365-2958.1992.tb01498.x. [DOI] [PubMed] [Google Scholar]
- Ullrich C., Kluge B., Palacz Z., Vater J. Cell-free biosynthesis of surfactin, a cyclic lipopeptide produced by Bacillus subtilis. Biochemistry. 1991 Jul 2;30(26):6503–6508. doi: 10.1021/bi00240a022. [DOI] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Vollenbroich D., Kluge B., D'Souza C., Zuber P., Vater J. Analysis of a mutant amino acid-activating domain of surfactin synthetase bearing a serine-to-alanine substitution at the site of carboxylthioester formation. FEBS Lett. 1993 Jul 5;325(3):220–224. doi: 10.1016/0014-5793(93)81077-d. [DOI] [PubMed] [Google Scholar]
- Vollenbroich D., Mehta N., Zuber P., Vater J., Kamp R. M. Analysis of surfactin synthetase subunits in srfA mutants of Bacillus subtilis OKB105. J Bacteriol. 1994 Jan;176(2):395–400. doi: 10.1128/jb.176.2.395-400.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker J. E., Saraste M., Runswick M. J., Gay N. J. Distantly related sequences in the alpha- and beta-subunits of ATP synthase, myosin, kinases and other ATP-requiring enzymes and a common nucleotide binding fold. EMBO J. 1982;1(8):945–951. doi: 10.1002/j.1460-2075.1982.tb01276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weckermann R., Fürbass R., Marahiel M. A. Complete nucleotide sequence of the tycA gene coding the tyrocidine synthetase 1 from Bacillus brevis. Nucleic Acids Res. 1988 Dec 23;16(24):11841–11841. doi: 10.1093/nar/16.24.11841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada M., Kurahashi K. Adenosine triphosphate and pyrophosphate dependent phenylalanine racemase of Bacillus brevis Nagano. J Biochem. 1968 Jan;63(1):59–69. doi: 10.1093/oxfordjournals.jbchem.a128748. [DOI] [PubMed] [Google Scholar]
- de Wet J. R., Wood K. V., DeLuca M., Helinski D. R., Subramani S. Firefly luciferase gene: structure and expression in mammalian cells. Mol Cell Biol. 1987 Feb;7(2):725–737. doi: 10.1128/mcb.7.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]