Abstract
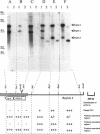
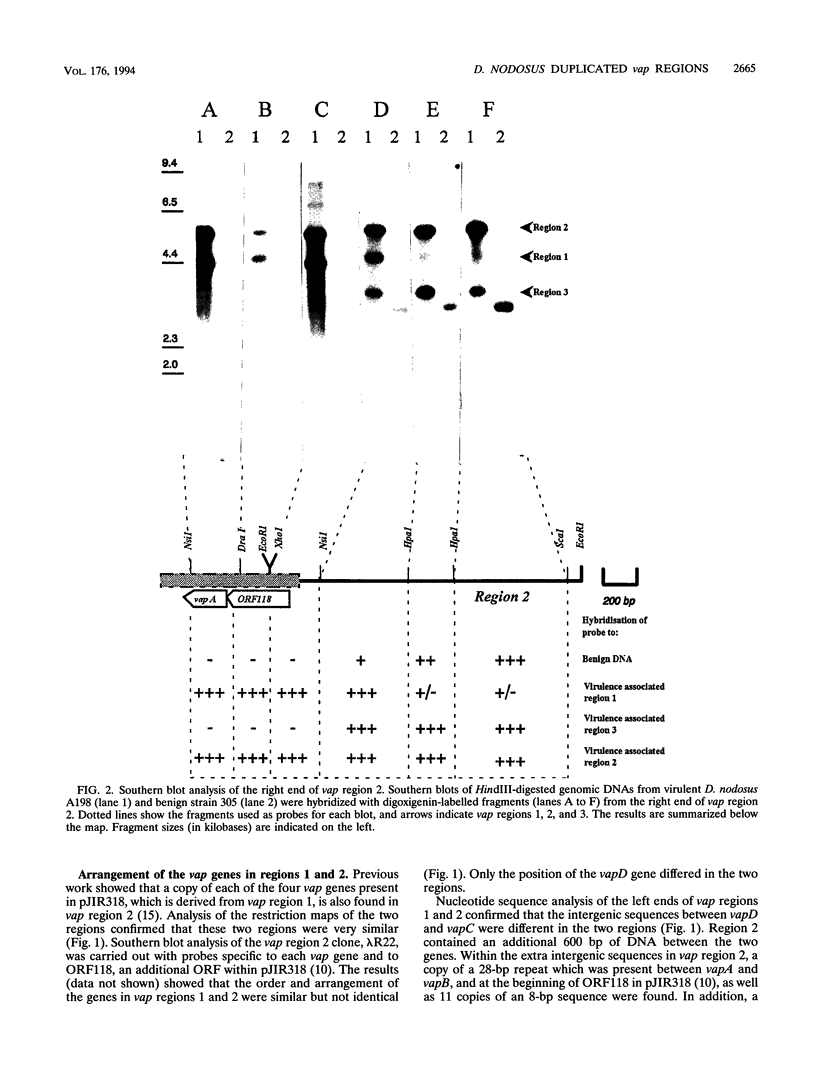
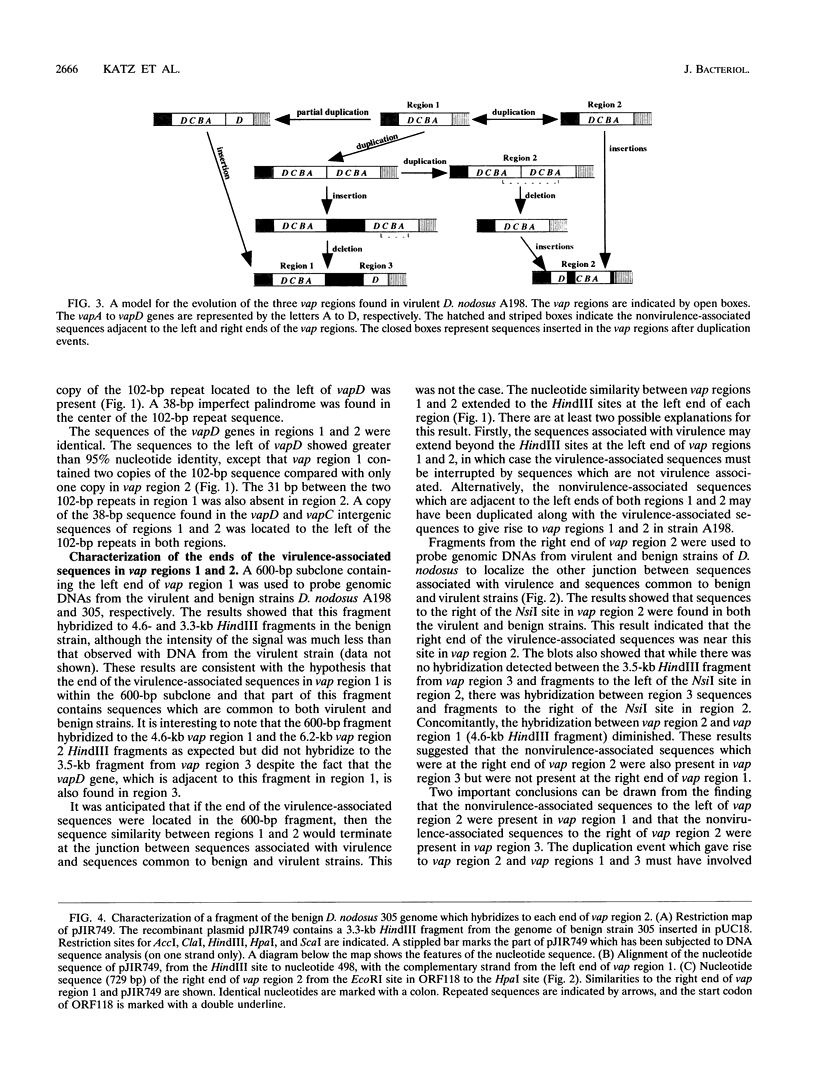
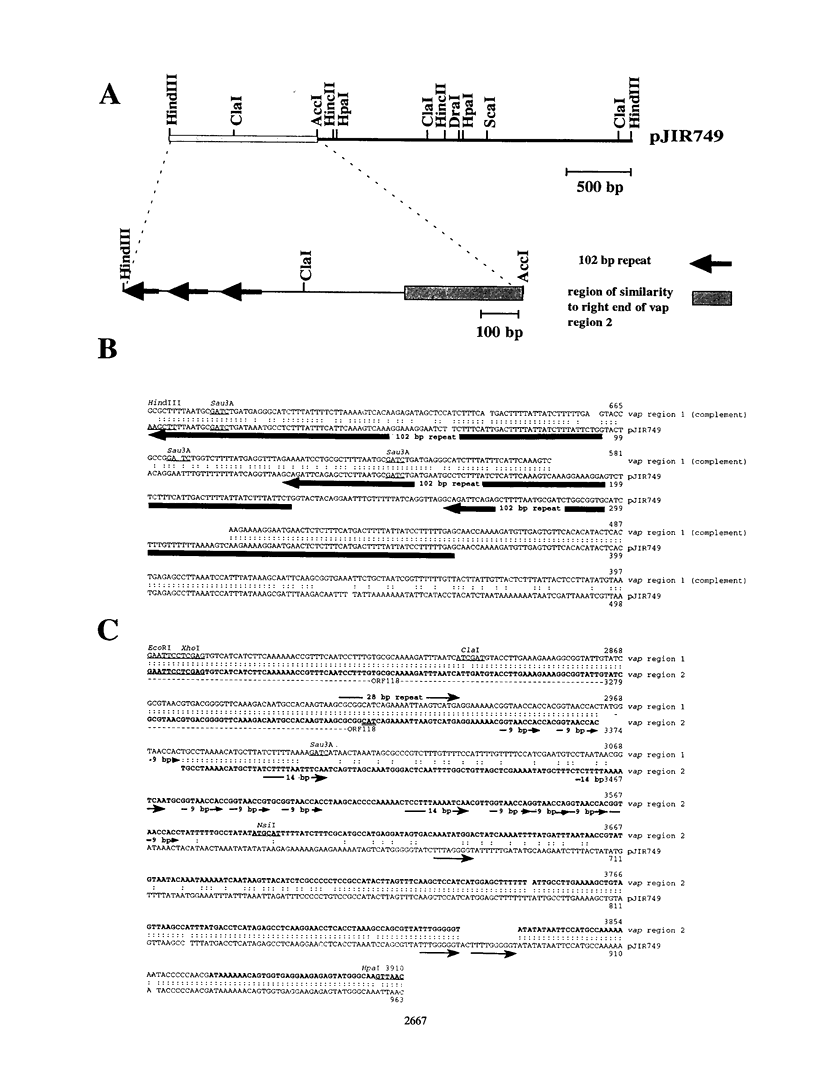
The recombinant plasmid pJIR318 contains a fragment of the Dichelobacter nodosus genome which is associated with virulence. Sequence analysis of the pJIR318 insert has shown that it contains four vap (virulence-associated protein) genes which are homologous to open reading frames found on the Escherichia coli F plasmid and the Neisseria gonorrhoeae cryptic plasmid (M. E. Katz, R. A. Strugnell, and J. I. Rood, Infect. and Immun. 60:4586-4592, 1992). The plasmid pJIR318 hybridizes to three regions of the D. nodosus genome, each of which has now been isolated. Regions 1 and 3 were found to be adjacent in the genome of D. nodosus A198, and the order of the vap genes in vap regions 1 and 2 were shown to be identical. Partial sequence analysis and Southern blot analysis of the vap regions showed that the three regions probably arose by a duplication event(s) followed by insertions and/or deletions. A recombinant plasmid, pJIR749, was isolated from a library of a benign D. nodosus strain, 305. This plasmid contained sequences from both ends of vap region 2. Analysis of pJIR749 showed that the sequences on either side of vap region 2 were separated by 324 bp in the genome of benign strain 305 and that the orientations of the sequences were different. It is clear that a simple insertion or deletion event did not generate the benign and virulent strains studied. A model which describes the evolution of the duplicated vap regions in D. nodosus A198 is presented.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson B. J., Bills M. M., Egerton J. R., Mattick J. S. Cloning and expression in Escherichia coli of the gene encoding the structural subunit of Bacteroides nodosus fimbriae. J Bacteriol. 1984 Nov;160(2):748–754. doi: 10.1128/jb.160.2.748-754.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Elleman T. C. Pilins of Bacteroides nodosus: molecular basis of serotypic variation and relationships to other bacterial pilins. Microbiol Rev. 1988 Jun;52(2):233–247. doi: 10.1128/mr.52.2.233-247.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frischauf A. M., Lehrach H., Poustka A., Murray N. Lambda replacement vectors carrying polylinker sequences. J Mol Biol. 1983 Nov 15;170(4):827–842. doi: 10.1016/s0022-2836(83)80190-9. [DOI] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz M. E., Howarth P. M., Yong W. K., Riffkin G. G., Depiazzi L. J., Rood J. I. Identification of three gene regions associated with virulence in Dichelobacter nodosus, the causative agent of ovine footrot. J Gen Microbiol. 1991 Sep;137(9):2117–2124. doi: 10.1099/00221287-137-9-2117. [DOI] [PubMed] [Google Scholar]
- Katz M. E., Strugnell R. A., Rood J. I. Molecular characterization of a genomic region associated with virulence in Dichelobacter nodosus. Infect Immun. 1992 Nov;60(11):4586–4592. doi: 10.1128/iai.60.11.4586-4592.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korch C., Hagblom P., Ohman H., Göransson M., Normark S. Cryptic plasmid of Neisseria gonorrhoeae: complete nucleotide sequence and genetic organization. J Bacteriol. 1985 Aug;163(2):430–438. doi: 10.1128/jb.163.2.430-438.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDougall J., Margarita D., Saint Girons I. Homology of a plasmid from the spirochete Treponema denticola with the single-stranded DNA plasmids. J Bacteriol. 1992 Apr;174(8):2724–2728. doi: 10.1128/jb.174.8.2724-2728.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skerman T. M. Determination of some in vitro growth requirements of Bacteroides nodosus. J Gen Microbiol. 1975 Mar;87(1):107–119. doi: 10.1099/00221287-87-1-107. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]