Abstract
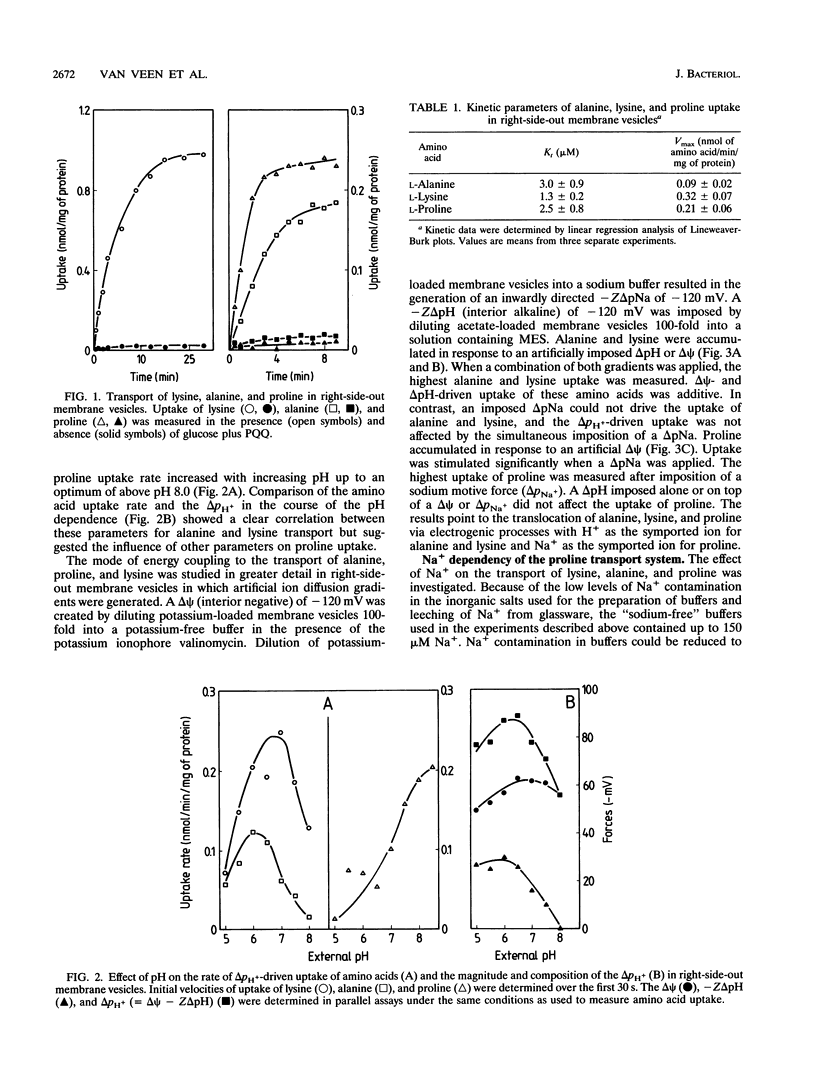
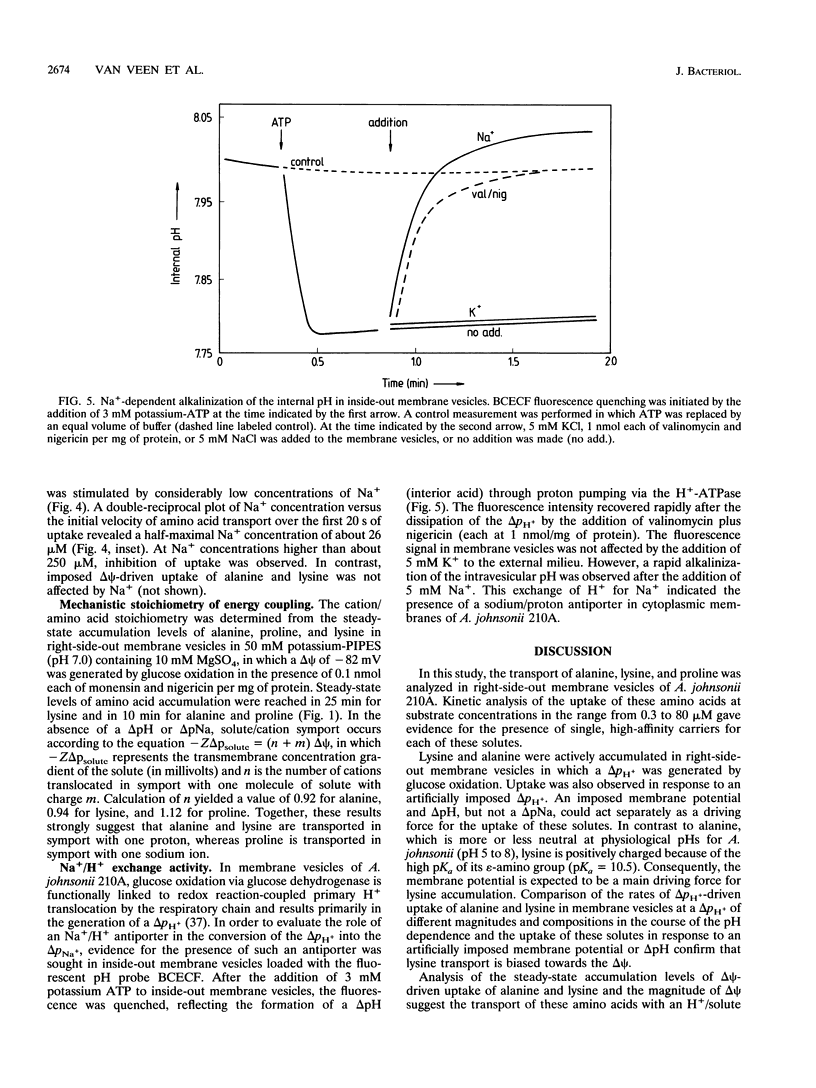
Amino acid transport in right-side-out membrane vesicles of Acinetobacter johnsonii 210A was studied. L-Alanine, L-lysine, and L-proline were actively transported when a proton motive force of -76 mV was generated by the oxidation of glucose via the membrane-bound glucose dehydrogenase. Kinetic analysis of amino acid uptake at concentrations of up to 80 microM revealed the presence of a single transport system for each of these amino acids with a Kt of less than 4 microM. The mode of energy coupling to solute uptake was analyzed by imposition of artificial ion diffusion gradients. The uptake of alanine and lysine was driven by a membrane potential and a transmembrane pH gradient. In contrast, the uptake of proline was driven by a membrane potential and a transmembrane chemical gradient of sodium ions. The mechanistic stoichiometry for the solute and the coupling ion was close to unity for all three amino acids. The Na+ dependence of the proline carrier was studied in greater detail. Membrane potential-driven uptake of proline was stimulated by Na+, with a half-maximal Na+ concentration of 26 microM. At Na+ concentrations above 250 microM, proline uptake was strongly inhibited. Generation of a sodium motive force and maintenance of a low internal Na+ concentration are most likely mediated by a sodium/proton antiporter, the presence of which was suggested by the Na(+)-dependent alkalinization of the intravesicular pH in inside-out membrane vesicles. The results show that both H+ and Na+ can function as coupling ions in amino acid transport in Acinetobacter spp.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambudkar S. V., Zlotnick G. W., Rosen B. P. Calcium efflux from Escherichia coli. Evidence for two systems. J Biol Chem. 1984 May 25;259(10):6142–6146. [PubMed] [Google Scholar]
- Anderson R. R., Menzel R., Wood J. M. Biochemistry and regulation of a second L-proline transport system in Salmonella typhimurium. J Bacteriol. 1980 Mar;141(3):1071–1076. doi: 10.1128/jb.141.3.1071-1076.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonucci T. K., Oxender D. L. The molecular biology of amino-acid transport in bacteria. Adv Microb Physiol. 1986;28:145–180. doi: 10.1016/s0065-2911(08)60238-6. [DOI] [PubMed] [Google Scholar]
- Booth I. R. Regulation of cytoplasmic pH in bacteria. Microbiol Rev. 1985 Dec;49(4):359–378. doi: 10.1128/mr.49.4.359-378.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown I. I., Galperin MYu, Glagolev A. N., Skulachev V. P. Utilization of energy stored in the form of Na+ and K+ ion gradients by bacterial cells. Eur J Biochem. 1983 Aug 1;134(2):345–349. doi: 10.1111/j.1432-1033.1983.tb07573.x. [DOI] [PubMed] [Google Scholar]
- Cairney J., Higgins C. F., Booth I. R. Proline uptake through the major transport system of Salmonella typhimurium is coupled to sodium ions. J Bacteriol. 1984 Oct;160(1):22–27. doi: 10.1128/jb.160.1.22-27.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castle A. M., Macnab R. M., Shulman R. G. Measurement of intracellular sodium concentration and sodium transport in Escherichia coli by 23Na nuclear magnetic resonance. J Biol Chem. 1986 Mar 5;261(7):3288–3294. [PubMed] [Google Scholar]
- Cook A. M., Fewson C. A. Evidence for specific transport mechanisms for aromatic compounds in bacterium N.C.I.B. 8250. Biochim Biophys Acta. 1972 Dec 1;290(1):384–388. doi: 10.1016/0005-2736(72)90081-8. [DOI] [PubMed] [Google Scholar]
- Driessen A. J. Secondary transport of amino acids by membrane vesicles derived from lactic acid bacteria. Antonie Van Leeuwenhoek. 1989 Aug;56(2):139–160. doi: 10.1007/BF00399978. [DOI] [PubMed] [Google Scholar]
- Driessen A. J., de Jong S., Konings W. N. Transport of branched-chain amino acids in membrane vesicles of Streptococcus cremoris. J Bacteriol. 1987 Nov;169(11):5193–5200. doi: 10.1128/jb.169.11.5193-5200.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driessen A. J., van Leeuwen C., Konings W. N. Transport of basic amino acids by membrane vesicles of Lactococcus lactis. J Bacteriol. 1989 Mar;171(3):1453–1458. doi: 10.1128/jb.171.3.1453-1458.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fewson C. A. Microbial metabolism of mandelate: a microcosm of diversity. FEMS Microbiol Rev. 1988 Apr-Jun;4(2):85–110. doi: 10.1111/j.1574-6968.1988.tb02737.x. [DOI] [PubMed] [Google Scholar]
- Grothe S., Krogsrud R. L., McClellan D. J., Milner J. L., Wood J. M. Proline transport and osmotic stress response in Escherichia coli K-12. J Bacteriol. 1986 Apr;166(1):253–259. doi: 10.1128/jb.166.1.253-259.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanada K., Yamato I., Anraku Y. Solubilization and reconstitution of proline carrier in Escherichia coli; quantitative analysis and optimal conditions. Biochim Biophys Acta. 1988 Apr 7;939(2):282–288. doi: 10.1016/0005-2736(88)90072-7. [DOI] [PubMed] [Google Scholar]
- Juni E. Genetics and physiology of Acinetobacter. Annu Rev Microbiol. 1978;32:349–371. doi: 10.1146/annurev.mi.32.100178.002025. [DOI] [PubMed] [Google Scholar]
- Kaback H. R., Barnes E. M., Jr Mechanisms of active transport in isolated membrane vesicles. II. The mechanism of energy coupling between D-lactic dehydrogenase and beta-galactoside transport in membrane preparations from Escherichia coli. J Biol Chem. 1971 Sep 10;246(17):5523–5531. [PubMed] [Google Scholar]
- Kaczorowski G. J., Kaback H. R. Mechanism of lactose translocation in membrane vesicles from Escherichia coli. 1. Effect of pH on efflux, exchange, and counterflow. Biochemistry. 1979 Aug 21;18(17):3691–3697. doi: 10.1021/bi00584a009. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Milner J. L., Grothe S., Wood J. M. Proline porter II is activated by a hyperosmotic shift in both whole cells and membrane vesicles of Escherichia coli K12. J Biol Chem. 1988 Oct 15;263(29):14900–14905. [PubMed] [Google Scholar]
- Molenaar D., Abee T., Konings W. N. Continuous measurement of the cytoplasmic pH in Lactococcus lactis with a fluorescent pH indicator. Biochim Biophys Acta. 1991 Nov 14;1115(1):75–83. doi: 10.1016/0304-4165(91)90014-8. [DOI] [PubMed] [Google Scholar]
- Ohyama T., Imaizumi R., Igarashi K., Kobayashi H. Escherichia coli is able to grow with negligible sodium ion extrusion activity at alkaline pH. J Bacteriol. 1992 Dec;174(23):7743–7749. doi: 10.1128/jb.174.23.7743-7749.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padan E., Schuldiner S. Intracellular pH regulation in bacterial cells. Methods Enzymol. 1986;125:337–352. doi: 10.1016/s0076-6879(86)25029-6. [DOI] [PubMed] [Google Scholar]
- Poolman B., Driessen A. J., Konings W. N. Regulation of arginine-ornithine exchange and the arginine deiminase pathway in Streptococcus lactis. J Bacteriol. 1987 Dec;169(12):5597–5604. doi: 10.1128/jb.169.12.5597-5604.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen B. P. Basic amino acid transport in Escherichia coli. J Biol Chem. 1971 Jun 10;246(11):3653–3662. [PubMed] [Google Scholar]
- Shinbo T., Kamo N., Kurihara K., Kobatake Y. A PVC-based electrode sensitive to DDA+ as a device for monitoring the membrane potential in biological systems. Arch Biochem Biophys. 1978 Apr 30;187(2):414–422. doi: 10.1016/0003-9861(78)90052-8. [DOI] [PubMed] [Google Scholar]
- Sonna L. A., Maloney P. C. Identification and functional reconstitution of phosphate: sugar phosphate antiport of Staphylococcus aureus. J Membr Biol. 1988 Mar;101(3):267–274. doi: 10.1007/BF01872841. [DOI] [PubMed] [Google Scholar]
- Steffes C., Ellis J., Wu J., Rosen B. P. The lysP gene encodes the lysine-specific permease. J Bacteriol. 1992 May;174(10):3242–3249. doi: 10.1128/jb.174.10.3242-3249.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Veen H. W., Abee T., Kortstee G. J., Konings W. N., Zehnder A. J. Characterization of two phosphate transport systems in Acinetobacter johnsonii 210A. J Bacteriol. 1993 Jan;175(1):200–206. doi: 10.1128/jb.175.1.200-206.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhoogt H. J., Smit H., Abee T., Gamper M., Driessen A. J., Haas D., Konings W. N. arcD, the first gene of the arc operon for anaerobic arginine catabolism in Pseudomonas aeruginosa, encodes an arginine-ornithine exchanger. J Bacteriol. 1992 Mar;174(5):1568–1573. doi: 10.1128/jb.174.5.1568-1573.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Veen H. W., Abee T., Kortstee G. J., Konings W. N., Zehnder A. J. Mechanism and energetics of the secondary phosphate transport system of Acinetobacter johnsonii 210A. J Biol Chem. 1993 Sep 15;268(26):19377–19383. [PubMed] [Google Scholar]



