Abstract
hemA and hemM, which are 213 bp apart and divergently transcribed, were separately cloned. We found that hemA is required for 5-aminolevulinic acid (ALA) synthesis in two ALA- auxotrophs. Overexpression of hemM alone did not produce ALA. More ALA was produced by strains harboring a plasmid with both hemA and hemM than by those with hemA alone. We conclude that hemA alone is required for ALA synthesis but hemA and hemM are required for maximal ALA synthesis.
Full text
PDF
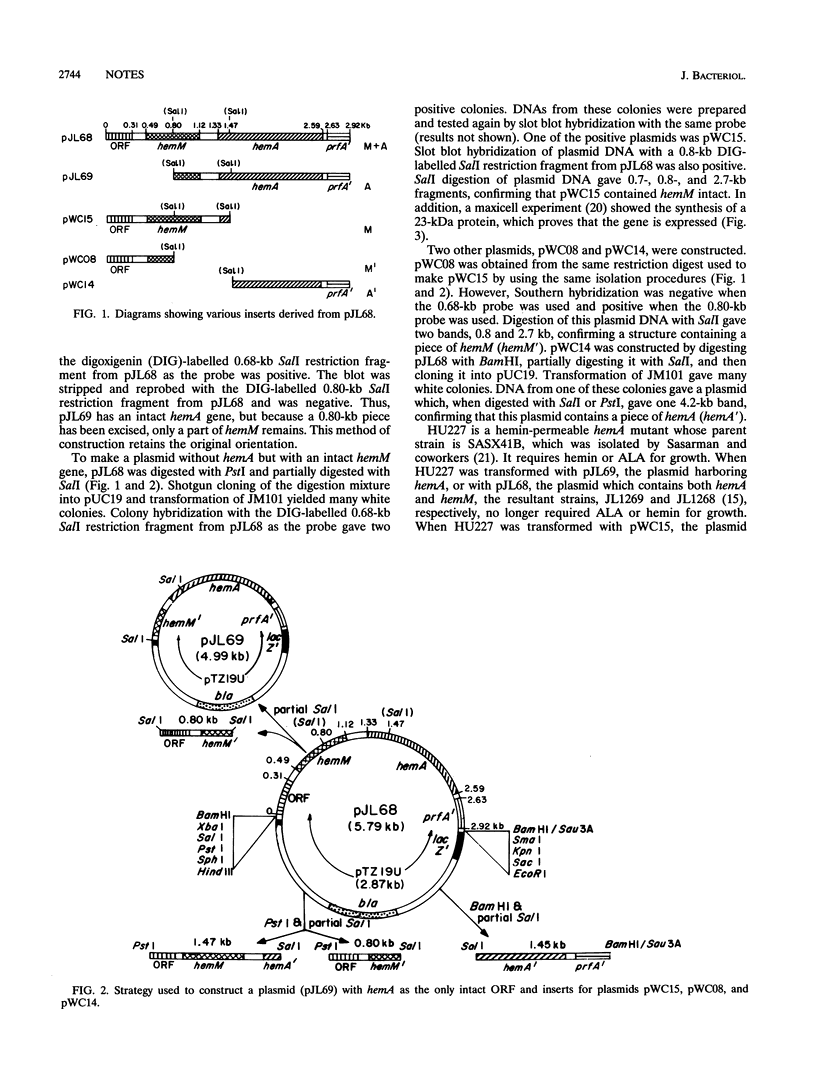
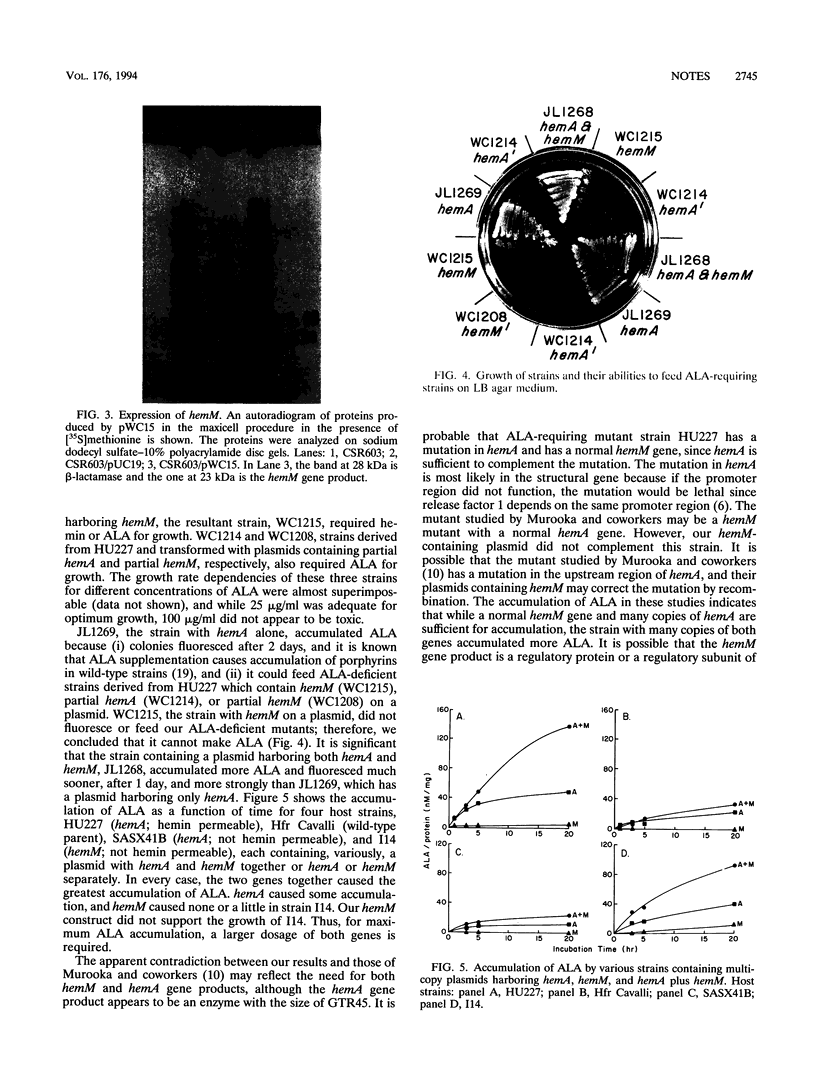

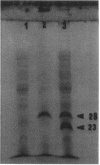
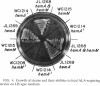
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avissar Y. J., Beale S. I. Biosynthesis of Tetrapyrrole Pigment Precursors : Formation and Utilization of Glutamyl-tRNA for delta-Aminolevulinic Acid Synthesis by Isolated Enzyme Fractions from Chlorella Vulgaris. Plant Physiol. 1988 Nov;88(3):879–886. doi: 10.1104/pp.88.3.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avissar Y. J., Beale S. I. Identification of the enzymatic basis for delta-aminolevulinic acid auxotrophy in a hemA mutant of Escherichia coli. J Bacteriol. 1989 Jun;171(6):2919–2924. doi: 10.1128/jb.171.6.2919-2924.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beale S. I., Castelfranco P. A. The Biosynthesis of delta-Aminolevulinic Acid in Higher Plants: I. Accumulation of delta-Aminolevulinic Acid in Greening Plant Tissues. Plant Physiol. 1974 Feb;53(2):291–296. doi: 10.1104/pp.53.2.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M. W., Jahn D., O'Neill G. P., Söll D. Purification of the glutamyl-tRNA reductase from Chlamydomonas reinhardtii involved in delta-aminolevulinic acid formation during chlorophyll biosynthesis. J Biol Chem. 1990 Mar 5;265(7):4058–4063. [PubMed] [Google Scholar]
- Drolet M., Péloquin L., Echelard Y., Cousineau L., Sasarman A. Isolation and nucleotide sequence of the hemA gene of Escherichia coli K12. Mol Gen Genet. 1989 Apr;216(2-3):347–352. doi: 10.1007/BF00334375. [DOI] [PubMed] [Google Scholar]
- Elliott T. Cloning, genetic characterization, and nucleotide sequence of the hemA-prfA operon of Salmonella typhimurium. J Bacteriol. 1989 Jul;171(7):3948–3960. doi: 10.1128/jb.171.7.3948-3960.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoober J. K., Kahn A., Ash D. E., Gough S., Kannangara C. G. Biosynthesis of delta-aminolevulinate in greening barley leaves. IX. Structure of the substrate, mode of gabaculine inhibition, and the catalytic mechanism of glutamate 1-semialdehyde aminotransferase. Carlsberg Res Commun. 1988;53(1):11–25. doi: 10.1007/BF02908411. [DOI] [PubMed] [Google Scholar]
- Huang D. D., Wang W. Y. Chlorophyll biosynthesis in Chlamydomonas starts with the formation of glutamyl-tRNA. J Biol Chem. 1986 Oct 15;261(29):13451–13455. [PubMed] [Google Scholar]
- Ikemi M., Murakami K., Hashimoto M., Murooka Y. Cloning and characterization of genes involved in the biosynthesis of delta-aminolevulinic acid in Escherichia coli. Gene. 1992 Nov 2;121(1):127–132. doi: 10.1016/0378-1119(92)90170-t. [DOI] [PubMed] [Google Scholar]
- Jahn D., Michelsen U., Söll D. Two glutamyl-tRNA reductase activities in Escherichia coli. J Biol Chem. 1991 Feb 5;266(4):2542–2548. [PubMed] [Google Scholar]
- Li J. M., Brathwaite O., Cosloy S. D., Russell C. S. 5-Aminolevulinic acid synthesis in Escherichia coli. J Bacteriol. 1989 May;171(5):2547–2552. doi: 10.1128/jb.171.5.2547-2552.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J. M., Russell C. S., Cosloy S. D. Cloning and structure of the hem A gene of Escherichia coli K-12. Gene. 1989 Oct 30;82(2):209–217. doi: 10.1016/0378-1119(89)90046-2. [DOI] [PubMed] [Google Scholar]
- O'Neill G. P., Chen M. W., Söll D. delta-Aminolevulinic acid biosynthesis in Escherichia coli and Bacillus subtilis involves formation of glutamyl-tRNA. FEMS Microbiol Lett. 1989 Aug;51(3):255–259. doi: 10.1016/0378-1097(89)90406-0. [DOI] [PubMed] [Google Scholar]
- O'Neill G. P., Peterson D. M., Schön A., Chen M. W., Söll D. Formation of the chlorophyll precursor delta-aminolevulinic acid in cyanobacteria requires aminoacylation of a tRNAGlu species. J Bacteriol. 1988 Sep;170(9):3810–3816. doi: 10.1128/jb.170.9.3810-3816.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philipp-Dormston W. K., Doss M. Over-production of porphyrins and heme in heterotrophic bacteria. Z Naturforsch C. 1975 May-Jun;30(3):425–426. doi: 10.1515/znc-1975-5-624. [DOI] [PubMed] [Google Scholar]
- Schneegurt M. A., Beale S. I. Characterization of the RNA Required for Biosynthesis of delta-Aminolevulinic Acid from Glutamate : Purification by Anticodon-Based Affinity Chromatography and Determination That the UUC Glutamate Anticodon Is a General Requirement for Function in ALA Biosynthesis. Plant Physiol. 1988 Feb;86(2):497–504. doi: 10.1104/pp.86.2.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schön A., Krupp G., Gough S., Berry-Lowe S., Kannangara C. G., Söll D. The RNA required in the first step of chlorophyll biosynthesis is a chloroplast glutamate tRNA. Nature. 1986 Jul 17;322(6076):281–284. doi: 10.1038/322281a0. [DOI] [PubMed] [Google Scholar]
- Săsărman A., Surdeanu M., Horodniceanu T. Locus determining the synthesis of delta-aminolevulinic acid in Escherichia coli K-12. J Bacteriol. 1968 Nov;96(5):1882–1884. doi: 10.1128/jb.96.5.1882-1884.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkamp E., Chelm B. K. Isolation, nucleotide sequence, and preliminary characterization of the Escherichia coli K-12 hemA gene. J Bacteriol. 1989 Sep;171(9):4728–4735. doi: 10.1128/jb.171.9.4728-4735.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkamp E., Jahn M., Jahn D., Kumar A. M., Söll D. Glutamyl-tRNA reductase from Escherichia coli and Synechocystis 6803. Gene structure and expression. J Biol Chem. 1992 Apr 25;267(12):8275–8280. [PubMed] [Google Scholar]
- Wang W. Y., Huang D. D., Stachon D., Gough S. P., Kannangara C. G. Purification, Characterization, and Fractionation of the delta-Aminolevulinic Acid Synthesizing Enzymes from Light-Grown Chlamydomonas reinhardtii Cells. Plant Physiol. 1984 Mar;74(3):569–575. doi: 10.1104/pp.74.3.569. [DOI] [PMC free article] [PubMed] [Google Scholar]