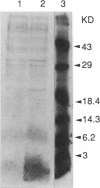
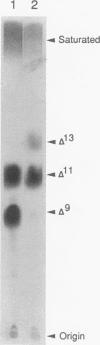
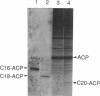
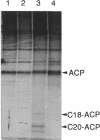
Abstract
The effects of inhibition of Escherichia coli phospholipid synthesis on the accumulation of intermediates of the fatty acid synthetic pathway have been previously investigated with conflicting results. We report construction of an E. coli strain that allows valid [14C]acetate labeling of fatty acids under these conditions. In this strain, acetate is a specific precursor of fatty acid synthesis and the intracellular acetate pools are not altered by blockage of phospholipid synthesis. By use of this strain, we show that significant pools of fatty acid synthetic intermediates and free fatty acids accumulate during inhibition of phospholipid synthesis and that the rate of synthesis of these intermediates is 10 to 20% of the rate at which fatty acids are synthesized during normal growth. Free fatty acids of abnormal chain length (e.g., cis-13-eicosenoic acid) were found to accumulate in glycerol-starved cultures. Analysis of extracts of [35S]methionine-labeled cells showed that glycerol starvation resulted in the accumulation of several long-chain acyl-acyl carrier protein (ACP) species, with the major species being ACP acylated with cis-13-eicosenoic acid. Upon the restoration of phospholipid biosynthesis, the abnormally long-chain acyl-ACPs decreased, consistent with transfer of the acyl groups to phospholipid. The introduction of multicopy plasmids that greatly overproduced either E. coli thioesterase I or E. coli thioesterase II fully relieved the inhibition of fatty acid synthesis seen upon glycerol starvation, whereas overexpression of ACP had no effect. Thioesterase I overproduction also resulted in disappearance of the long-chain acyl-ACP species. The release of inhibition by thiosterase overproduction, together with the correlation between the inhibition of fatty acid synthesis and the presence of abnormally long-chain acyl-ACPs, suggests with that these acyl-ACP species may act as feedback inhibitors of a key fatty acid synthetic enzyme(s).
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chang Y. Y., Cronan J. E., Jr Mapping nonselectable genes of Escherichia coli by using transposon Tn10: location of a gene affecting pyruvate oxidase. J Bacteriol. 1982 Sep;151(3):1279–1289. doi: 10.1128/jb.151.3.1279-1289.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho H., Cronan J. E., Jr Escherichia coli thioesterase I, molecular cloning and sequencing of the structural gene and identification as a periplasmic enzyme. J Biol Chem. 1993 May 5;268(13):9238–9245. [PubMed] [Google Scholar]
- Cronan J. E., Jr The unsaturated fatty acids of Escherichia coli. Biochim Biophys Acta. 1967 Dec 5;144(3):695–697. doi: 10.1016/0005-2760(67)90063-x. [DOI] [PubMed] [Google Scholar]
- Cronan J. E., Jr, Weisberg L. J., Allen R. G. Regulation of membrane lipid synthesis in Escherichia coli. Accumulation of free fatty acids of abnormal length during inhibition of phospholipid synthesis. J Biol Chem. 1975 Aug 10;250(15):5835–5840. [PubMed] [Google Scholar]
- Henry M. F., Cronan J. E., Jr A new mechanism of transcriptional regulation: release of an activator triggered by small molecule binding. Cell. 1992 Aug 21;70(4):671–679. doi: 10.1016/0092-8674(92)90435-f. [DOI] [PubMed] [Google Scholar]
- Ishiguro N., Sato G., Sasakawa C., Danbara H., Yoshikawa M. Identification of citrate utilization transposon Tn3411 from a naturally occurring citrate utilization plasmid. J Bacteriol. 1982 Mar;149(3):961–968. doi: 10.1128/jb.149.3.961-968.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackowski S., Rock C. O. Regulation of coenzyme A biosynthesis. J Bacteriol. 1981 Dec;148(3):926–932. doi: 10.1128/jb.148.3.926-932.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maloy S. R., Bohlander M., Nunn W. D. Elevated levels of glyoxylate shunt enzymes in Escherichia coli strains constitutive for fatty acid degradation. J Bacteriol. 1980 Aug;143(2):720–725. doi: 10.1128/jb.143.2.720-725.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maloy S. R., Nunn W. D. Role of gene fadR in Escherichia coli acetate metabolism. J Bacteriol. 1981 Oct;148(1):83–90. doi: 10.1128/jb.148.1.83-90.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre T. M., Bell R. M. Mutants of Escherichia coli defective in membrane phospholipid synthesis. Effect of cessation of net phospholipid synthesis on cytoplasmic and outer membranes. J Biol Chem. 1975 Dec 10;250(23):9053–9059. [PubMed] [Google Scholar]
- Mindich L. Control of fatty acid synthesis in bacteria. J Bacteriol. 1972 Apr;110(1):96–102. doi: 10.1128/jb.110.1.96-102.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naggert J., Narasimhan M. L., DeVeaux L., Cho H., Randhawa Z. I., Cronan J. E., Jr, Green B. N., Smith S. Cloning, sequencing, and characterization of Escherichia coli thioesterase II. J Biol Chem. 1991 Jun 15;266(17):11044–11050. [PubMed] [Google Scholar]
- Nunn W. D., Kelly D. L., Stumfall M. Y. Regulation of fatty acid synthesis during the cessation of phospholipid biosynthesis in Escherichia coli. J Bacteriol. 1977 Nov;132(2):526–531. doi: 10.1128/jb.132.2.526-531.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post-Beittenmiller D., Jaworski J. G., Ohlrogge J. B. In vivo pools of free and acylated acyl carrier proteins in spinach. Evidence for sites of regulation of fatty acid biosynthesis. J Biol Chem. 1991 Jan 25;266(3):1858–1865. [PubMed] [Google Scholar]
- Rawlings M., Cronan J. E., Jr The gene encoding Escherichia coli acyl carrier protein lies within a cluster of fatty acid biosynthetic genes. J Biol Chem. 1992 Mar 25;267(9):5751–5754. [PubMed] [Google Scholar]
- Rock C. O., Jackowski S. Regulation of phospholipid synthesis in Escherichia coli. Composition of the acyl-acyl carrier protein pool in vivo. J Biol Chem. 1982 Sep 25;257(18):10759–10765. [PubMed] [Google Scholar]
- Shen Z., Fice D., Byers D. M. Preparation of fatty-acylated derivatives of acyl carrier protein using Vibrio harveyi acyl-ACP synthetase. Anal Biochem. 1992 Jul;204(1):34–39. doi: 10.1016/0003-2697(92)90135-t. [DOI] [PubMed] [Google Scholar]
- Spencer A. K., Greenspan A. D., Cronan J. E., Jr Thioesterases I and II of Escherichia coli. Hydrolysis of native acyl-acyl carrier protein thioesters. J Biol Chem. 1978 Sep 10;253(17):5922–5926. [PubMed] [Google Scholar]
- Srere P. A. The citrate enzymes: their structures, mechanisms, and biological functions. Curr Top Cell Regul. 1972;5:229–283. doi: 10.1016/b978-0-12-152805-8.50013-7. [DOI] [PubMed] [Google Scholar]
- Takeshita S., Sato M., Toba M., Masahashi W., Hashimoto-Gotoh T. High-copy-number and low-copy-number plasmid vectors for lacZ alpha-complementation and chloramphenicol- or kanamycin-resistance selection. Gene. 1987;61(1):63–74. doi: 10.1016/0378-1119(87)90365-9. [DOI] [PubMed] [Google Scholar]
- Wu H. C., Lai J. S., Hayashi S., Giam C. Z. Biogenesis of membrane lipoproteins in Escherichia coli. Biophys J. 1982 Jan;37(1):307–315. doi: 10.1016/S0006-3495(82)84679-1. [DOI] [PMC free article] [PubMed] [Google Scholar]