Abstract
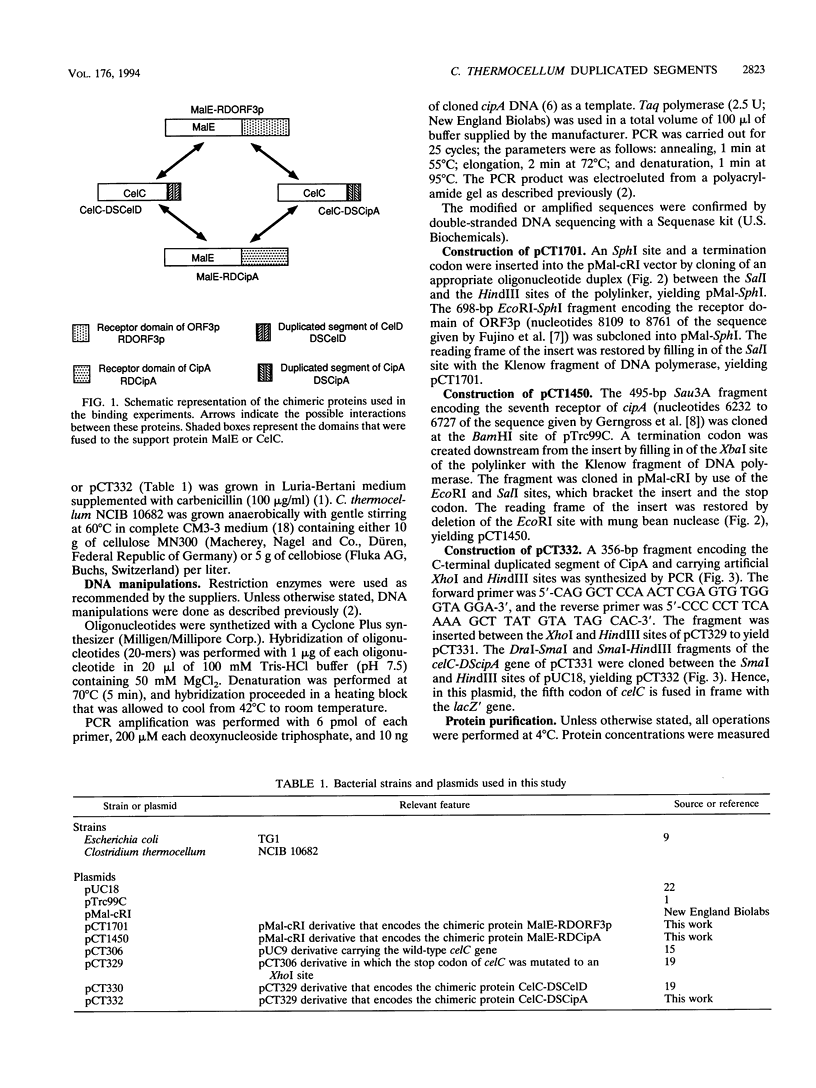
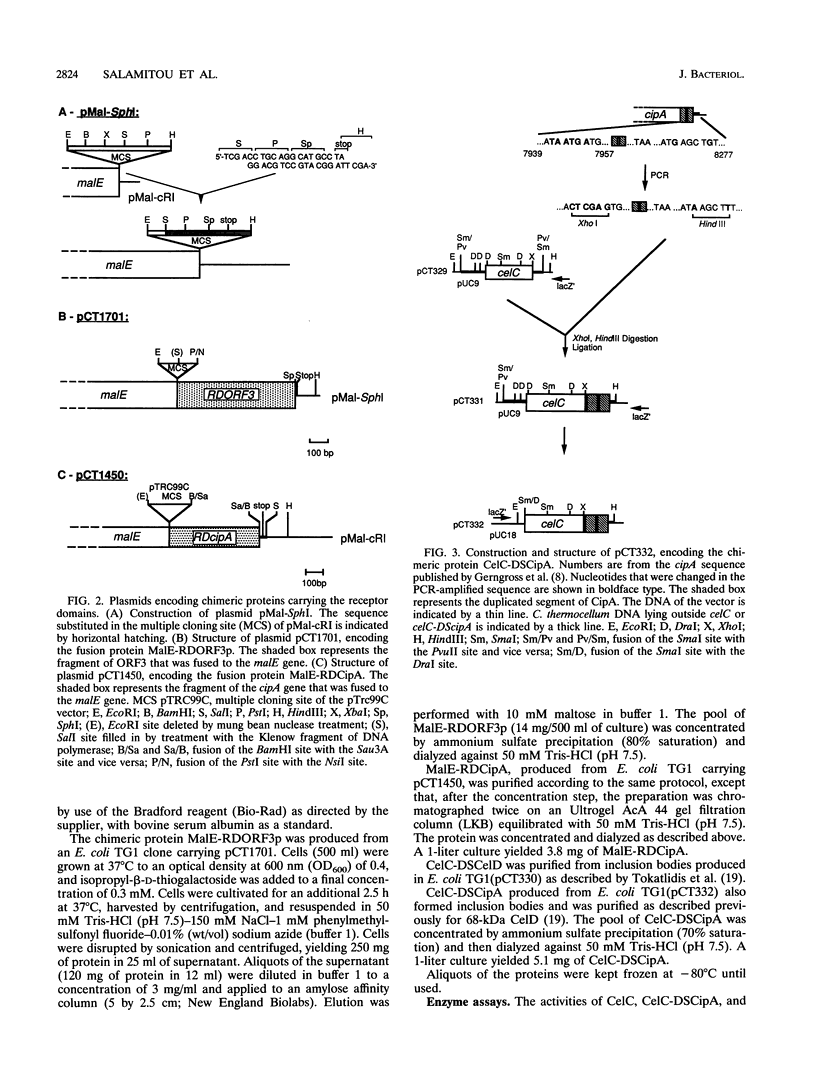
The binding specificity of the duplicated segments borne by Clostridium thermocellum endoglucanase CelD and by the cellulosome-integrating protein CipA was investigated. The fusion protein CelC-DSCelD, in which the duplicated segment of CelD was fused to the COOH terminus of endoglucanase CelC, bound with an affinity of 4.7 x 10(7) M-1 to the fusion protein MalE-RDCipA, in which the seventh receptor domain of CipA was grafted onto the COOH terminus of the Escherichia coli maltose-binding protein MalE. The affinity of CelC-DSCelD for the homologous chimeric protein MalE-RDORF3p, carrying the receptor of the surface protein ORF3p, was 6.9 x 10(6) M-1. The fusion protein CelC-DSCipA, in which the duplicated segment of CipA was grafted onto the COOH terminus of CelC, did not bind detectably to MalE-RDCipA or MalE-RDORF3p. However, Western blotting (immunoblotting) experiments indicated that the duplicated segment of CipA was able to bind to a set of C. thermocellum proteins which are different from those recognized by the duplicated segment of CelD. These results argue against the hypothesis that ORF3p interacts with the duplicated segment of CipA. More probably, ORF3p binds to individual cellulases and hemicellulases harboring duplicated segments.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amann E., Ochs B., Abel K. J. Tightly regulated tac promoter vectors useful for the expression of unfused and fused proteins in Escherichia coli. Gene. 1988 Sep 30;69(2):301–315. doi: 10.1016/0378-1119(88)90440-4. [DOI] [PubMed] [Google Scholar]
- Béguin P. Detection of cellulase activity in polyacrylamide gels using Congo red-stained agar replicas. Anal Biochem. 1983 Jun;131(2):333–336. doi: 10.1016/0003-2697(83)90178-1. [DOI] [PubMed] [Google Scholar]
- Chauvaux S., Beguin P., Aubert J. P., Bhat K. M., Gow L. A., Wood T. M., Bairoch A. Calcium-binding affinity and calcium-enhanced activity of Clostridium thermocellum endoglucanase D. Biochem J. 1990 Jan 1;265(1):261–265. doi: 10.1042/bj2650261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friguet B., Chaffotte A. F., Djavadi-Ohaniance L., Goldberg M. E. Measurements of the true affinity constant in solution of antigen-antibody complexes by enzyme-linked immunosorbent assay. J Immunol Methods. 1985 Mar 18;77(2):305–319. doi: 10.1016/0022-1759(85)90044-4. [DOI] [PubMed] [Google Scholar]
- Fujino T., Béguin P., Aubert J. P. Cloning of a Clostridium thermocellum DNA fragment encoding polypeptides that bind the catalytic components of the cellulosome. FEMS Microbiol Lett. 1992 Jul 1;73(1-2):165–170. doi: 10.1016/0378-1097(92)90602-k. [DOI] [PubMed] [Google Scholar]
- Fujino T., Béguin P., Aubert J. P. Organization of a Clostridium thermocellum gene cluster encoding the cellulosomal scaffolding protein CipA and a protein possibly involved in attachment of the cellulosome to the cell surface. J Bacteriol. 1993 Apr;175(7):1891–1899. doi: 10.1128/jb.175.7.1891-1899.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerngross U. T., Romaniec M. P., Kobayashi T., Huskisson N. S., Demain A. L. Sequencing of a Clostridium thermocellum gene (cipA) encoding the cellulosomal SL-protein reveals an unusual degree of internal homology. Mol Microbiol. 1993 Apr;8(2):325–334. doi: 10.1111/j.1365-2958.1993.tb01576.x. [DOI] [PubMed] [Google Scholar]
- Grépinet O., Chebrou M. C., Béguin P. Purification of Clostridium thermocellum xylanase Z expressed in Escherichia coli and identification of the corresponding product in the culture medium of C. thermocellum. J Bacteriol. 1988 Oct;170(10):4576–4581. doi: 10.1128/jb.170.10.4576-4581.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lamed R., Setter E., Bayer E. A. Characterization of a cellulose-binding, cellulase-containing complex in Clostridium thermocellum. J Bacteriol. 1983 Nov;156(2):828–836. doi: 10.1128/jb.156.2.828-836.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navas J., Béguin P. Site-directed mutagenesis of conserved residues of Clostridium thermocellum endoglucanase CelC. Biochem Biophys Res Commun. 1992 Dec 15;189(2):807–812. doi: 10.1016/0006-291x(92)92274-2. [DOI] [PubMed] [Google Scholar]
- Poole D. M., Morag E., Lamed R., Bayer E. A., Hazlewood G. P., Gilbert H. J. Identification of the cellulose-binding domain of the cellulosome subunit S1 from Clostridium thermocellum YS. FEMS Microbiol Lett. 1992 Dec 1;78(2-3):181–186. doi: 10.1016/0378-1097(92)90022-g. [DOI] [PubMed] [Google Scholar]
- Salamitou S., Lemaire M., Fujino T., Ohayon H., Gounon P., Béguin P., Aubert J. P. Subcellular localization of Clostridium thermocellum ORF3p, a protein carrying a receptor for the docking sequence borne by the catalytic components of the cellulosome. J Bacteriol. 1994 May;176(10):2828–2834. doi: 10.1128/jb.176.10.2828-2834.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamitou S., Tokatlidis K., Béguin P., Aubert J. P. Involvement of separate domains of the cellulosomal protein S1 of Clostridium thermocellum in binding to cellulose and in anchoring of catalytic subunits to the cellulosome. FEBS Lett. 1992 Jun 8;304(1):89–92. doi: 10.1016/0014-5793(92)80595-8. [DOI] [PubMed] [Google Scholar]
- Tailliez P., Girard H., Millet J., Beguin P. Enhanced Cellulose Fermentation by an Asporogenous and Ethanol-Tolerant Mutant of Clostridium thermocellum. Appl Environ Microbiol. 1989 Jan;55(1):207–211. doi: 10.1128/aem.55.1.207-211.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokatlidis K., Dhurjati P., Béguin P. Properties conferred on Clostridium thermocellum endoglucanase CelC by grafting the duplicated segment of endoglucanase CelD. Protein Eng. 1993 Nov;6(8):947–952. doi: 10.1093/protein/6.8.947. [DOI] [PubMed] [Google Scholar]
- Tokatlidis K., Dhurjati P., Millet J., Béguin P., Aubert J. P. High activity of inclusion bodies formed in Escherichia coli overproducing Clostridium thermocellum endoglucanase D. FEBS Lett. 1991 Apr 22;282(1):205–208. doi: 10.1016/0014-5793(91)80478-l. [DOI] [PubMed] [Google Scholar]
- Tokatlidis K., Salamitou S., Béguin P., Dhurjati P., Aubert J. P. Interaction of the duplicated segment carried by Clostridium thermocellum cellulases with cellulosome components. FEBS Lett. 1991 Oct 21;291(2):185–188. doi: 10.1016/0014-5793(91)81279-h. [DOI] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]