Abstract
We examined the capacity of the naturally occurring inhibitors of transforming growth factor β (TGF-β), decorin and latency associated peptide (LAP), to reverse depressed T cell functions in peripheral blood mononuclear cells (PBMCs) from patients with pulmonary tuberculosis (TB) in vitro and to counteract the suppressive properties of TGF-β on mycobacterial replication in blood monocytes (MN) in vitro. T cell blastogenesis in response to purified protein derivative (PPD) in PBMCs of TB patients that were cocultured with decorin or LAP reached levels comparable to those observed in healthy tuberculin-responsive control subjects. Decorin and LAP were as effective as neutralizing antibody to TGF-β in correcting depressed T cell proliferation. Coculture of PBMCs from healthy PPD reactive individuals with neutralizing antibody to TGF-β, decorin, or LAP did not affect T cell blastogenesis. Levels of interferon-γ in cultures of PPD-stimulated PBMCs from patients with TB increased by more than 2-fold in the presence of maximal concentrations of either of the inhibitors of TGF-β, whereas TGF-β immunoreactivity declined to background levels. Coculture with optimal concentrations of decorin or LAP also led to reductions in mycobacterial growth in MN infected with Mycobacterium tuberculosis (MTB) in vitro by 51% and 62%, respectively, when compared with cells left untreated. In parallel, levels of immunoreactive TGF-β in MTB-infected MN cultures containing decorin or LAP decreased to background levels. These data indicate that the naturally occurring inhibitors of TGF-β, decorin and LAP, efficiently abrogate the suppressive effects of TGF-β in PBMCs of TB patients and in MN infected with MTB in vitro. Therefore, these agents may be considered as adjuncts to antituberculous chemotherapy, and may be particularly useful in treatment of TB that is unresponsive to conventional chemotherapy.
Keywords: blastogenesis, macrophage effector function, decorin, latency associated peptide of transforming growth factor β
In the past decade, the incidence of tuberculosis (TB) has been increasing worldwide despite continuing efforts to improve diagnosis and treatment. Recent research has identified a complex interaction between Mycobacterium tuberculosis (MTB) and the host mononuclear cells that may culminate in suppression of protective immune responses. Knowledge of these immunopathogenic circuits may allow better targeting of biological modulators as adjuncts in the treatment of TB. Adjunctive therapy may be particularly useful in drug-resistant TB and in TB in the immunodeficient host.
Transforming growth factor β1 (TGF-β), a pluripotent cytokine that suppresses T cell responses (1–4) and deactivates macrophage effector functions (5–7), is induced in blood monocytes (MN) by MTB (8) and its purified protein derivative (PPD) (9). Also, the major secretory protein of growing mycobacteria, 30-kDa α antigen (10), and the major mycobacterial cell wall lipoglycan, lipoarabinomannan (LAM) (11) induce MN production of TGF-β. TGF-β is expressed in Langhans’ giant cells and epithelioid cells in tuberculous pulmonary granulomas and in MN of patients with TB (12). Recently, MN were shown to be more potent in production of TGF-β than alveolar macrophages (13). Additionally, studies of bronchoalveolar cells of TB patients indicate an alveolitis, characterized by an abundance of immature mononuclear phagocytes, likely representing MN recently recruited from the blood to the infected focus (14). Therefore, TGF-β may well be represented at sites of active MTB infection in the lung and contribute to the immunopathogenesis of TB locally.
Excess TGF-β underlies the depressed in vitro T cell responses to MTB antigens in patients with TB, as coculture of peripheral blood mononuclear cells (PBMCs) from TB patients with neutralizing antibody to TGF-β normalizes T cell blastogenesis and enhances production of interferon γ (IFN-γ) (10). IFN-γ appears to play a central role in limiting MTB infection, as recently shown in the murine models of TB (15, 16). Additionally, TGF-β augments mycobacterial replication in MTB-infected human MN in vitro, and blocks IFN-γ-mediated macrophage-effector function against MTB (8) and Mycobacterium avium (17). We have recently observed that MN of patients with active TB produce high levels of TGF-β that appears to be in a mature, biologically active form, readily suppressing T cell functions (C.S.H., J.J.E., R. Hussain, F. Shahid, and Z.T., unpublished work). Thus, agents that counteract the effects of TGF-β by improving both T cell and macrophage effector functions may be helpful as adjuncts to antituberculous therapy.
Two inhibitors of TGF-β, latency associated peptide (LAP) and decorin bind bioactive TGF-β and neutralize its activity. As opposed to neutralizing antibody to TGF-β, which is synthetic, both decorin and LAP occur naturally and as such may be involved in the physiological regulation of production and effects of TGF-β. By binding to excess bioactive TGF-β at disease sites, the natural inhibitors of TGF-β have the potential to limit the deleterious effects of the cytokine. LAP is part of the latent TGF-β complex (18). Dissolution of noncovalent links between LAP and the 25-kDa homodimer of active TGF-β leads to activation of the cytokine (18, 19). Following this “activation,” however, LAP may reassociate with active TGF-β, thus rendering it biologically inactive (20). Decorin is a 45-kDa proteoglycan (21) abundant in extracellular matrix, such as cartilage and connective tissue. Decorin has been shown previously to exert a beneficial effect in a rat model of glomerulonephritis in which TGF-β is known to play a major immunopathogenic role (22). Inactivation of TGF-β bioactivity by decorin is thought to involve binding of the decorin core protein to the active TGF-β molecule (23). Decorin is expressed along with TGF-β in pulmonary tissue of rats with bleomycin-induced pulmonary fibrosis (24) and may play a role in counteracting the profibrotic tendencies of the TGF-β.
In this study, we showed that LAP and decorin are potent inhibitors of the effects of TGF-β both on MTB replication in MN and on T cell proliferative responses and IFN-γ production of PBMCs of patients with active pulmonary TB in vitro. Furthermore, these inhibitors of TGF-β did not significantly affect T cell responses of healthy tuberculin reactors. These data indicate that binding of TGF-β by its inhibitors to form inactive complexes may be an efficient means of abrogating the effects of the cytokine on T cells and macrophages, thus arresting immunosuppressive circuits that are initiated or maintained by TGF-β.
MATERIALS AND METHODS
Human Subjects.
After obtaining informed consent, patients with active pulmonary TB and healthy tuberculin reactors were studied. A diagnosis of TB in the patients was established by routine radiographic, clinical, and bacteriological criteria at MetroHealth Medical Center (Cleveland). Patients who had received antituberculous therapy for more than 8 weeks and patients with factors potentially affecting the immune response were excluded: (i) age >70 years; (ii) concomitant debilitating diseases such as cancer, diabetes, or treatment with immunosuppressive drugs; and (iii) positive serology for HIV. The diagnosis of TB was based on the demonstration of acid-fast bacilli in sputum and was eventually confirmed by positive culture in all patients. By radiographic standards of the National Tuberculosis and Respiratory Disease Association (25) all patients studied had either moderately advanced or far advanced disease. MN from a separate group of healthy individuals were studied in MTB growth inhibition experiments.
Antigens and Antibodies.
PPD, a gift from Lederle (American Cyanamid) was used at a final concentration of 10 μg/ml. Neutralizing antibody to TGF-β and LAP (R&D Diagnostics, Minneapolis) were used at a final concentration of 10 μg/ml and 1–100 ng/ml, respectively. Bovine decorin, a gift of L. Rosenberg (Montefiore Medical Center, New York) was used at a final concentration of 0.5–50 μg/ml. Recombinant human TGF-β1 (R&D Diagnostics) was used at a final concentration of 10 ng/ml. The endotoxin content of all reagents was tested by the Limulus amoebocyte lysate assay (BioWhittaker) and was <0.01 ng/mg protein for each reagent.
Preparation of Cells and Generation of Cytokine Containing Supernatants.
On the day of the study, 30 ml of blood was collected in heparinized syringes from patients and tuberculin reactive control subjects. PBMCs were obtained by sedimentation over Ficoll–Hypaque (Pharmacia) as described (8). To induce cytokines, PBMCs suspended in Iscove’s modified Dulbecco’s medium (IMDM) (BioWhittaker) containing 50 units/ml penicillin, 50 μg/ml streptomycin, 2 mM glutamine (Sigma), supplemented with 2% of pooled human serum (PHS) were incubated at 1 × 106 cells/ml for 72 h. Culture supernatants were collected and stored at −70°C until use.
Blastogenesis.
PBMCs from patients or control subjects were suspended in RPMI 1640 medium (BioWhittaker) containing 10% fetal calf serum and incubated in triplicate (105 cells per well) in 96-well round bottom microtiter plates (Falcon) with or without PPD in the presence of antibody to TGF-β, LAP, decorin, or media for 5 days. Cells then were pulsed with [3H]thymidine [1 μCi per well (1 Ci = 37 GBq); specific activity 6.7 Ci/mmol; ICN] and harvested 18–24 h later. Incorporation of radioactivity was measured by scintillation spectroscopy. Results are expressed as stimulation index [(mean counts of triplicate cultures − background) : background].
Isolation of MN and Assay of MTB Growth Inhibition.
MN were obtained from PBMCs from four healthy individuals (two were PPD positive and two were PPD negative) by adherence to plastic Petri dishes precoated with ≈1 ml of pooled human serum (PHS) for 1 h at 37°C. Plates then were washed three times with prewarmed RPMI 1640 medium to remove nonadherent cells, covered with 5 ml of Hanks’ balanced salt solution without calcium or magnesium (BioWhittaker), and incubated at 4°C for 20 min. Adherent cells were removed by scraping with a plastic cell scraper, sedimented by centrifugation at 250 × g for 10 min, and the resulting pellet was suspended in RPMI 1640 medium containing 2% autologous serum. Cells obtained in this manner were 90–94% peroxidase positive, 80–90% nonspecific esterase positive, and <1% granulocytes by Wright’s stain and are referred to as MN. Viability of MN as determined by exclusion of 0.2% Trypan blue was >95% in all experiments.
For the MTB growth inhibition assay, MN were placed in 96-well round bottom microtiter wells (1 × 105 cells per well) and allowed to readhere for 1 h. Cells then were infected with MTB (H37Ra) at a ratio of 50:1 (MTB:target cell). Clumping of mycobacteria was controlled by vigorous vortexing (15 min) and sonication (20 s) of the mycobacterial solution immediately prior to infection. After an incubation period of 1 h, to allow internalization of mycobacteria, uningested bacteria were removed by washing vigorously with RPMI 1640 medium. The infected MN monolayers then were cultured in triplicate with medium with or without the addition of decorin, LAP, or neutralizing antibody to TGF-β for up to 7 days. Mycobacterial replication in cells lysed at days 0, 4, and 7 was assessed in the colony-forming unit (cfu) assay as described (8).
Immunoassays for Cytokines.
IFN-γ immunoreactivity was assessed with a commercially available ELISA (Endogen, Cambridge, MA), which is sensitive to 15 pg/ml of IFN-γ. The ELISA for TGF-β (10) uses a mouse monoclonal antibody to TGF-β 1,2,3 (Genzyme) as capture antibody and a polyclonal chicken anti-human TGF-β 1 antibody (R&D Diagnostics) as capping antibody. All samples were acid activated prior to determination of TGF-β immunoreactivity. This assay will detect 0.15 ng/ml of TGF-β immunoreactivity.
Competition of Binding of [125I]TGF-β to Decorin and LAP.
This assay was performed as described by Yamaguchi et al. (23). Briefly, 96-well microtiter wells were coated overnight with decorin or LAP. [125I]TGF-β (5 × 105 cpm − specific activity 2,000–4,500 Ci/mmol; NEN), alone or in combination with various concentrations of decorin, LAP, or the control protein BSA, were added to the wells and the plates incubated for 4 h at 37°C. The bound radioactivity was solubilized in 1% SDS with 0.3% NaOH, and radioactivity in each sample was assessed. Effects of the inhibitors of TGF-β on binding of radioactively labeled cytokine were expressed as percent reduction of total binding of TGF-β.
Mink Lung Cell (Mv1Lu) Assay for TGF-β Bioactivity.
The Mv1Lu assay for TGF-β bioactivity was employed as described (12). Briefly, Mv1Lu cells (American Type Culture Collection) were seeded into 96-well microtiter plates (Falcon) (2 × 104 cells per well) and allowed to adhere for 8 h. Crude culture supernatants from TB patients were added (50% vol/vol) in triplicate to the wells containing the Mv1Lu cells and incubated at 37°C for 18 h. [3H]Thymidine (1 μCi per well, ICN) was added for the final 5 h of culture. Incorporation of radioactivity then was assessed by scintillation spectroscopy. A standard curve was generated from wells containing recombinant TGF-β (0.005–2 ng/ml) alone or preincubated with neutralizing antibody to TGF-β. In this assay, 2 ng of recombinant TGF-β suppresses cell proliferation by 90% and neutralizing antibody (5 μg/ml) reverses this suppression completely.
Statistical Analysis.
Data were analyzed using the Student’s t test, paired t test, and linear correlation and regression analysis. P ≤ 0.05 was considered significant.
RESULTS
Natural Inhibitors of TGF-β Interfere with Specific Binding of [125I]TGF-β.
We first evaluated the capacity of decorin and LAP to inhibit the binding of radioactively labeled TGF-β in a competitive assay. Briefly, 96-well microtiter plates were coated with either decorin or LAP and then incubated with [125I]TGF-β (5 × 105 cpm per well) alone or in combination with increasing concentrations of decorin, LAP, or BSA. Nonspecific binding was assessed in wells containing 100-fold molar excess of unlabeled TGF-β. The bound radioactivity in each well was solubilized and counted in a γ counter. In the two experiments performed, total binding of [125I]-labeled TGF-β alone was 13.5 and 16%, respectively, on decorin-coated plates, and 15 and 16%, respectively, on LAP-coated plates. Binding to decorin-coated plates was comparable to what has been reported before (23). Coculture of [125I]TGF-β with increasing concentrations of either decorin or LAP as competitor led to reductions in binding of radioactively labeled TGF-β in a dose-responsive manner, reaching a maximum of 71% (mean of two separate experiments) with decorin (50 μg/ml) and 76% with LAP (100 ng/ml). The control protein, BSA, did not inhibit the binding of [125I]TGF-β to either decorin- or LAP-coated microtiter plates (Table 1).
Table 1.
Competition of [125I]TGF-β binding to decorin- and LAP-coated microtiter plates
| Precoating | Competitor used | % reduction of binding of [125I]TGF-β
|
|
|---|---|---|---|
| Exp. 1 | Exp. 2 | ||
| Decorin | Decorin | 78 | 63 |
| BSA | 16 | 11 | |
| LAP | LAP | 60 | 92 |
| BSA | 13 | 11 | |
Samples containing [125I]TGF-β alone or in combination with decorin (50 μg/ml), LAP (100 ng/ml), or BSA (50 μg/ml) were added to microtiter plates precoated with decorin or LAP. The bound radioactivity was solubilized and counted in a γ counter. Results represent percent reduction of binding of [125I]TGF-β in two experiments performed.
Enhancement of T Cell Blastogenic Responses of Patients with TB and Healthy Controls by Inhibitors of TGF-β.
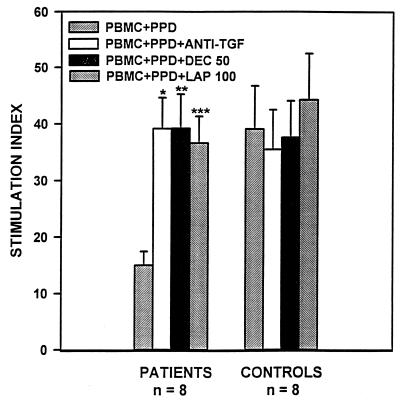
We next assessed the effect of LAP and decorin on T cell responses of patients with newly diagnosed pulmonary TB. PBMCs from TB patients and tuberculin-reactive control subjects were stimulated with or without PPD in the presence or absence of anti-TGF-β antibody, decorin, or LAP. In the absence of PPD, decorin or LAP or antibody to TGF-β did not affect proliferation in PBMCs of patients or control subjects (data not shown). Furthermore, coculture with anti-TGF-β, decorin, or LAP did not affect PPD-stimulated T cell blastogenesis in healthy control subjects. By contrast, PPD-stimulated T cell blastogenesis in PBMCs from TB patients was enhanced in the presence of anti-TGF-β antibody or increasing concentrations of decorin or LAP. Decorin (50 μg/ml) led to a 2.6-fold increase in T cell responses (P < 0.002, n = 8) and LAP (100 ng/ml) led to a 2.5-fold augmentation in blastogenesis in PBMCs of TB patients (P < 0.001, n = 8) (Fig. 1). Increases in T cell responses were comparable to those observed with neutralizing antibody to TGF-β (10 μg/ml) (2.6-fold; P < 0.0001, n = 8) (Fig. 1). In fact, T cell blastogenesis in PBMCs of patients cocultured with neutralizing antibody to TGF-β or maximal amounts of either inhibitor was comparable to those observed in healthy tuberculin reactors.
Figure 1.
Effects of decorin and LAP on PPD-induced blastogenesis in TB patients. PBMCs from TB patients and control subjects were cultured for 6 days in 96-well microtiter plates in medium alone or medium containing decorin (50 μg/ml), LAP (100 ng/ml), or neutralizing antibody to TGF-β (10 μg/ml), in the absence and presence of PPD (10 μg/ml). [3H]Thymidine was present for the final 18 h of culture and incorporation of radioactivity was assessed by scintillation spectroscopy. Results are expressed as stimulation indices. ∗, P ≤ 0.0001; ∗∗, P ≤ 0.002; ∗∗∗, P ≤ 0.001, n = 8, when compared with stimulation index in PBMCs cultured in medium containing PPD alone.
Effects of Inhibitors of TGF-β on Cytokine Levels in PBMC Cultures.
We next measured PPD-stimulated production of the cytokines IFN-γ and TGF-β in PBMC cultures of TB patients and control subjects. PBMCs of TB patients and controls were cultured for 72 h in the presence or absence of PPD with or without decorin, LAP, or neutralizing antibody to TGF-β. In the absence of PPD, decorin or LAP or neutralizing antibody to TGF-β did not induce production of IFN-γ in PBMCs of patients or control subjects (data not shown). PPD-induced IFN-γ immunoreactivity in culture supernatants of patients were depressed to 1/10th of the activity found in supernatants of healthy controls (Table 2). On the other hand, concentrations of TGF-β were increased by 2.5-fold (Table 2) in PBMC culture supernatants of TB patients as compared with control subjects. PPD-stimulated production of IFN-γ in PBMC cultures from control subjects was not affected by decorin or LAP. By contrast, PPD-stimulated IFN-γ levels in PBMCs from TB patients improved in a dose-dependent manner when increasing concentrations of decorin or LAP were present for the duration of culture (data not shown). IFN-γ immunoreactivity increased by 2.5-fold in supernatants of PBMCs from TB patients that were cocultured with maximal concentrations of decorin (50 μg/ml) and by 2.9-fold when PBMCs were cultured in the presence of maximal concentrations of LAP (100 ng/ml) (P ≤ 0.005 and 0.03, respectively, n = 8) (Table 2). Concentrations of TGF-β in PBMC supernatants from TB patients cultured in the presence of decorin or LAP, on the other hand, decreased in a dose-dependent manner and were as low as background levels when maximal concentrations of either inhibitor were present during culture (P ≤ 0.004, n = 8 for both) (Table 2). Reductions of TGF-β immunoreactivity in PBMC culture supernatants prepared in the presence of maximal amounts of decorin or LAP showed an inverse correlation with improved T cell responses and IFN-γ production [blastogenesis (decorin): r = −0.761, P ≤ 0.03; (LAP): r = −0.746, P ≤ 0.03, respectively; n = 8 for both; IFN (decorin): r = −0.714, P ≤ 0.03; (LAP): −0.764, P ≤ 0.03, n = 8)]. Decorin or LAP did not affect TNF-α or interleukin (IL) 10 concentrations of PPD-stimulated or -unstimulated PBMC culture supernatants (data not shown). Thus, the effects of the naturally occurring inhibitors of TGF-β on production of IFN-γ were not secondary to TGF-β-mediated effects on the production of other cytokines implicated in the immunopathogenesis of TB.
Table 2.
Production of IFN-γ and TGF-β by PBMCs of TB patients and control subjects cocultured with optimal concentrations of antibody to TGF-β, decorin, or LAP
| Stimulus | TB patients
|
Controls
|
||
|---|---|---|---|---|
| IFN-γ | TGF-β | IFN-γ | TGF-β | |
| NIL | <0.03 | 1.3 ± 0.5 | <0.03 | 0.7 ± 0.3 |
| PPD | 0.224 ± 0.08 | 13.5 ± 3.6* | 2.7 ± 0.6 | 5.3 ± 1.3 |
| PPD + NAB | 0.442 ± 0.12† | 3.8 ± 1.3‡ | 2.6 ± 0.5 | 2.2 ± 1.3 |
| PPD + DEC | 0.556 ± 0.15† | 4.1 ± 3.6§ | 2.5 ± 0.5 | 2.2 ± 1.2 |
| PPD + LAP | 0.803 ± 0.22¶ | 3.9 ± 2.3§ | 2.8 ± 0.5 | 1.7 ± 1.3 |
PBMCs of TB patients and healthy tuberculin-reactive control subjects were cultured for 72 h with or without PPD (10 μg/ml) in the presence or absence of neutralizing antibody to TGF-β (NAB) (10 μg/ml), decorin (DEC) (50 μg/ml), or LAP (100 ng/ml). IFN-γ and TGF-β immunoreactivities in culture supernatants were assessed by ELISA and both are expressed as ng/ml. Results represent mean ± SE, n = 8.
P ≤ 0.0008 compared with TGF-β in supernatants of unstimulated PBMCs from TB patients.
P ≤ 0.005 compared with IFN-γ in supernatants of PPD-stimulated PBMCs from TB patients.
P ≤ 0.006 compared with TGF-β in supernatants of PBMCs from TB patients cultured with PPD alone.
P ≤ 0.004 compared with TGF-β in supernatants of PBMCs from TB patients cultured with PPD alone.
P ≤ 0.03 compared with IFN-γ in supernatants of PPD-stimulated PBMCs from TB patients.
Biologically Active TGF-β in Crude PBMC Culture Supernatants of TB Patients; Effects of Decorin and LAP.
Suppression of T cell blastogenesis and IFN-γ production in TB cultures and their restitution by coculture with both inhibitors of TGF-β suggest that at least some of the TGF-β present in PBMC culture supernatants from TB patients is in the bioactive form. To assess this possibility, we next measured TGF-β bioactivity in PBMC supernatants from TB patients and healthy tuberculin reactive controls using the Mv1Lu assay. DNA synthesis in these cells is suppressed by mature bioactive TGF-β in a dose-responsive manner and reaches 90% in the presence of recombinant TGF-β at 2 ng/ml (12).
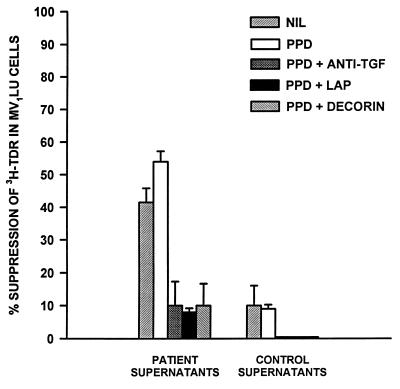
PPD-stimulated and -unstimulated PBMC supernatants from TB patients and healthy PPD-positive subjects were added (50% vol/vol) in triplicate to wells containing Mv1Lu cells. PBMC culture supernatants of healthy tuberculin reactive controls (PPD stimulated or not) did not affect incorporation of [3H]thymidine into Mv1Lu cells significantly (≈90% of baseline activity; Fig. 2), indicating that all of the TGF-β activity (as determined by ELISA, Table 2) in these supernatants is latent. By contrast, PPD-stimulated PBMC culture supernatants from TB patients depressed [3H]thymidine incorporation in Mv1Lu cells to 46 ± 6% (mean ± SE, n = 4)) of baseline activity (Fig. 2). Furthermore, even unstimulated culture supernatants from TB patients suppressed DNA synthesis in Mv1Lu cells, suggesting spontaneous production and activation of TGF-β in PBMCs of TB patients. The presence of decorin or LAP during culture of PBMCs from TB patients, on the other hand, decreased the suppressive effects of culture supernatants on Mv1Lu cells by 80% (Fig. 2). Thus, cultures of PBMCs from TB patients (with and without PPD stimulation) contain bioactive TGF-β, and this activity is abrogated by TGF-β inhibitors.
Figure 2.
Effects of decorin or LAP on TGF-β bioactivity in supernatants of PBMCs from TB patients. PBMCs from TB patients and healthy tuberculin-reactive control subjects were cultured in medium alone and medium containing PPD alone or together with decorin (50 μg/ml) or LAP (100 ng/ml). Culture supernatants were obtained after 72 h and added at 50% (vol/vol) to Mv1Lu cells and incubated overnight. [3H]Thymidine was added for the final 5 h of culture and incorporation of radioactivity was assessed by scintillation spectroscopy. Results are expressed as percent suppression (mean ± SE, n = 4) of DNA synthesis in Mv1Lu cells. Decorin and LAP reversed suppression in Mv1Lu cells by supernatants of PBMCs of TB patients.
Natural Inhibitors of TGF-β Slow Growth of MTB in Vitro.
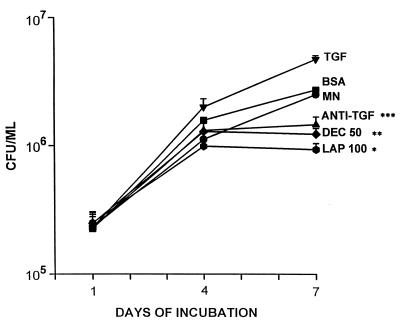
We have previously shown that exogenous TGF-β, as well as TGF-β produced by MN infected with MTB in vitro, leads to accelerated mycobacterial replication, and that addition of neutralizing antibody to TGF-β augments the capacity of MN to limit growth of MTB (8). We therefore examined the effects of the naturally occurring inhibitors of TGF-β decorin (0.5–50 μg/ml) and LAP (1–100 ng/ml) on mycobacterial replication in direct comparison with neutralizing antibody to the cytokine. Coculture with increasing concentrations of either inhibitor led to a stepwise decrease in MTB growth in infected MN. The addition of maximal concentrations of decorin (50 μg/ml) or LAP (100 ng/ml) led to reductions in mycobacterial growth by 51 and 62%, respectively, when compared with untreated cells (P ≤ 0.006 and 0.0001; n = 4), whereas coculture with neutralizing antibody to TGF-β (10 μg/ml) resulted in a decrease of mycobacterial replication by 42% (P ≤ 0.01; n = 4) (Fig. 3). As shown previously (8), coculture of MTB-infected MN with recombinant TGF-β (10 ng/ml) doubled mycobacterial growth (P ≤ 0.01; n = 4) (Fig. 3). MTB-induced TGF-β immunoreactivity in MN culture supernatants collected on day 7 of the cfu assay decreased in a dose-dependent fashion with increasing amounts of decorin or LAP present for the duration of culture. TGF-β immunoreactivity decreased from 5.68 ± 0.90 ng/ml to 0.62 ± 0.22 ng/ml (LAP, 100 ng/ml) and 0.50 ± 0.19 ng/ml (decorin, 50 μg/ml), respectively, when maximal amounts of either inhibitor were added to the culture system [P ≤ 0.02 (decorin) and P ≤ 0.005 (LAP)] (data not shown). Viability of cells did not differ between the wells that contained medium alone or wells containing LAP, decorin, neutralizing antibody to TGF-β, or recombinant TGF-β for the duration of culture. By day 7 of culture, 80–85% of MN remained adherent to the plates.
Figure 3.
Effects of decorin and LAP on mycobacterial replication in MN in vitro. MN were infected with MTB (H37Ra) and cultured for up to 7 days in medium alone or medium containing optimal concentrations of antibody to TGF-β (anti-TGF-β) (10 μg/ml), decorin (DEC) (50 μg/ml), LAP (100 ng/ml), BSA (50 mg/ml), or recombinant TGF-β (10 ng/ml). Mycobacterial replication was assessed in the cfu assay. Results represent mean ± SE cfu/ml, n = 4. *, P ≤ 0.0001; **, P ≤ 0.006; ***, P ≤ 0.01 as compared with mycobacterium-infected MN cultured in medium alone (MN).
DISCUSSION
MTB and its major protein and polysaccharide constituents are potent inducers of production of cytokines in mononuclear phagocytes, some of which, such as TGF-β, are immunosuppressive and macrophage deactivating (8–11, 26–28). The recruitment of MN to sites of active MTB infection and activation of MN to production of cytokines, in particular TGF-β, may be important in the immunopathogenesis of TB. Autoinduction of TGF-β may be key in maintaining high levels of the cytokine in situ, thus perpetuating immunosuppressive circuits. Modulation of excess TGF-β activity may assume particular importance in shifting the cytokine milieu of the tuberculous lesion to one that is dominated by macrophage activation and immunoprotection. Results of this study indicate that the naturally occurring inhibitors of TGF-β, decorin, and LAP, can effectively abrogate immunosuppressive effects of TGF-β in PBMCs of TB patients and reverse TGF-β-mediated macrophage deactivation in vitro. Therefore, these agents may be considered as potential adjuncts to antituberculous chemotherapy.
TGF-β, a product of activated MN, exhibits a wide spectrum of immunomodulatory functions (29), including down-regulation of production of proinflammatory cytokines such as IL-1, IL-6, and TNF-α (4, 30), suppression of production of IFN-γ (31), inhibition of T cell and B cell mitogenesis (1–4, 30, 32), attenuation of generation and cytotoxicity of natural killer cells and T cells (33–35), and modulation of expression of cell surface receptors such as HLA-DR and Fcγ-receptor III (36, 37). In recent years, TGF-β also has been linked to the pathogenesis of a number of diseases, both due to intracellular pathogens and of inflammatory nature (38). In murine models of Leishmania amazonensis and Trypanosoma cruzi infection, TGF-β enhanced replication of either parasite both in vitro and in vivo (39–42). Furthermore, there is strong evidence for a role for TGF-β in diseases characterized by accumulation of excess amounts of extracellular matrix such as mesangial injury in the rat model of glomerulonephritis (22), diabetic nephropathy in humans (43), cirrhosis of the liver both in rats and humans (38), and in muscular fibrosis seen in patients with Duchenne muscular dystrophy (44). Overproduction of TGF-β also has been linked to fibrotic pulmonary processes such as bleomycin-induced pulmonary fibrosis in rats (24, 45), as well as lung pathology in humans with idiopathic pulmonary fibrosis (46) and sarcoidosis (47).
Recent studies indicate that TB should be added to the growing list of diseases associated with overexpression of TGF-β. MN of healthy individuals produce TGF-β in response to MTB and its components PPD and LAM (8, 9, 11). LAM, a major constituent of MTB cell walls, may be present in large quantities in the lung of individuals with reactivation pulmonary TB. LAM may particularly favor the induction of TGF-β over other cytokines (11). Furthermore, TGF-β produced by MTB-infected MN promotes intracellular mycobacterial replication and counteracts macrophage-activating cytokines such as TNF-α and IFN-γ (8). Thus, a vicious cycle may be initiated, whereby production of TGF-β and mycobacterial growth amplify one another. TGF-β is expressed in tuberculous granulomas in the lung as well as by MN of patients with active pulmonary TB (12). Recently, we have shown that coculture of PBMCs of TB patients with neutralizing antibody to TGF-β restored blastogenesis in response to PPD, but not to the control antigen candida, to levels found in healthy tuberculin reactors, and improved PPD-induced IFN-γ production (10). This study again supports the notion that TGF-β is linked to suppressed T cell responses in TB, because PPD-stimulated PBMC culture supernatants from patients with TB contained significant amounts of immunoreactive and bioactive TGF-β. In addition, the natural inhibitors of TGF-β, decorin and LAP, corrected the suppressed T cell blastogenic responses of TB patients to levels comparable to those seen in healthy controls, and significantly improved the production of IFN-γ in PBMCs of patients. In fact, the effects of decorin and LAP in reversing the immunosuppressive properties of TGF-β equalled and even surpassed that of neutralizing antibody to TGF-β.
The above findings attain particular importance when considering the physiology of activation of TGF-β. TGF-β is secreted by cells as a biologically inactive complex of the 25-kDa homodimer of active TGF-β bound noncovalently to LAP and latent TGF-β binding protein (18). TGF-β can only interact with its receptor once dissociated from LAP and latent TGF-β binding protein (activated TGF-β). Activation of TGF-β may require interaction with proteolytic enzymes such as plasmin (18) or alterations of the carbohydrate structure of LAP by glycosidases or sialidases (19). Alveolar macrophages of rabbits injected with bacillus Calmette–Guérin demonstrate increased sialidase activity (48); and sialidase generates active TGF-β from its latent form (49). Furthermore, extremes of pH (acidic or alkali) are effective in activating TGF-β in vitro (18). Thus, the acidic, protease-rich milieu of the tuberculous granuloma may also be conducive to activation of TGF-β. We have recently shown that crude MTB antigen-induced MN supernatants from patients with active pulmonary TB contained high concentrations of bioactive TGF-β (C.S.H., J.J.E., R. Hussain, F. Shahid, and Z.T., unpublished work). When MN supernatants of TB patients were added to PBMCs of healthy PPD-responsive donors these supernatants displayed TGF-β-mediated suppressive properties on production of PPD-induced IL-2 and IFN-γ without a prior need for activation in vitro. In this study, both unstimulated and PPD-stimulated PBMC culture supernatants from TB patients contained bioactive and immunoreactive TGF-β. By contrast, unstimulated and PPD-simulated PBMC culture supernatants from healthy tuberculin reactive-control subjects contained minimal amounts of biologically active TGF-β. Because LAP and decorin only associate with active TGF-β, excess quantities of either molecule may bind and inactivate mature TGF-β. In fact, the concentrations of both decorin and LAP leading to optimal reversal of depressed T cell functions were well in molar excess (4-fold for LAP and 1000-fold for decorin) of the concentration of TGF-β present in PPD-stimulated PBMC culture supernatants.
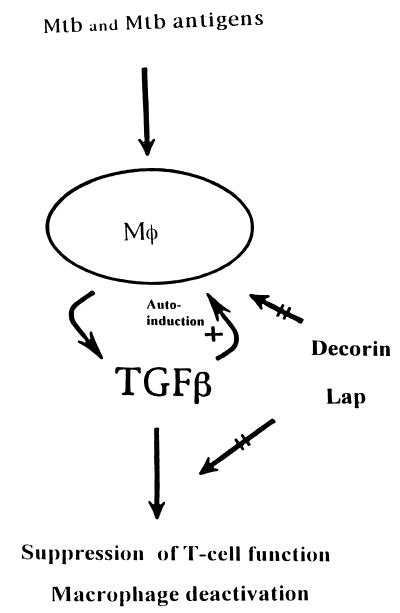
Considering the above findings, decorin and LAP would be particularly useful as immunoadjuvants at sites of active disease where excess bioactive TGF-β is present, such as the tuberculous lesions of pulmonary TB. As shown schematically in Fig. 4, presumably at sites of active MTB infection, decorin and LAP may counteract TGF-β-mediated T cell suppression and macrophage deactivation, and may interrupt autoregulatory circuits initiated and maintained by TGF-β. In support of this contention, in a rat model of glomerulonephritis in which TGF-β is thought to be pathogenic, daily intravenous administration of decorin for 4 and 6 consecutive days prevented excessive collagen deposition in the glomeruli and accelerated recovery in diseased animals (22). Expression of decorin, on the other hand, was down-regulated in fibrotic lung tissue in bleomycin-induced pulmonary fibrosis in the rat, a condition associated with large increases in mRNA for TGF-β (22). These findings suggest that interactions between TGF-β and decorin may be instrumental to the balance between pro- and anti-inflammatory influences regulating pulmonary fibrosis. Furthermore, recently, LAP administered intraperitoneally to transgenic mice overexpressing TGF-β has been shown to counteract the effects of TGF-β on hepatocellular proliferation (50).
Figure 4.
Schematic representation of the potential effects of the naturally occurring inhibitors of TGF-β, decorin and LAP, on TGF-β activity in situ. Presumably at sites of active MTB infection, such as tuberculous granulomas, TGF-β is produced by the mononuclear phagocytes (Mφ), which are infected with MTB and/or exposed to MTB antigens. Through positive autoregulation initiated by TGF-β itself (+), high tissue levels of the cytokine are maintained. Excess TGF-β undermines antimycobacterial defenses by suppression of T cell function and deactivation of macrophages. Decorin and LAP counteract (straight arrows) the effects of TGF-β and thereby interrupt both autoinduction and immunosuppression.
Data presented in this study provide evidence for a role of decorin and LAP in regulating the effects of TGF-β in human cell culture systems: both molecules restored T cell blastogenesis and improved IFN-γ responses in PBMCs from TB patients and reversed TGF-β-mediated macrophage deactivation in MN from healthy individuals in vitro. These properties provide a strong rationale for considering either TGF-β inhibitor for immunotherapy of TB. Since these agents only affect bioactive TGF-β, their effects may be restricted to sites of active MTB infection where excess TGF-β is expected. Evaluating the efficacy of decorin and LAP in animal models of TB would be important in determining the in vivo usefulness of these molecules. These in vivo studies are necessary for any future development of TGF-β inhibitors as adjuncts to antituberculous therapy in human TB. Immunotherapies may be particularly useful in the treatment of multidrug-resistant TB and TB in the immunocompromised host.
Acknowledgments
This work was supported by grants from the U.S. Public Health Service (AI-18471) and (HL-51636) and a merit review grant from the Department of Veterans Affairs.
ABBREVIATIONS
- TB
tuberculosis
- TGF-β
transforming growth factor β
- MTB
Mycobacterium tuberculosis
- PBMCs
peripheral blood mononuclear cells
- MN
blood monocytes
- LAP
latency associated peptide
- IFN-γ
interferon-γ
- PPD
purified protein derivative
- LAM
lipoarabinomannan
- IL
interleukin
- cfu
colony-forming unit(s)
References
- 1.Kehrl J H, Wakefield L M, Roberts A B, Jakowlew S, Alvarez-Mon M, Derynck R, Sporn M B, Fauci A S. J Exp Med. 1986;163:1037–1050. doi: 10.1084/jem.163.5.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shalaby M R, Ammann A J. Cell Immunol. 1988;112:343–350. doi: 10.1016/0008-8749(88)90303-6. [DOI] [PubMed] [Google Scholar]
- 3.Wahl S M, Hunt D A, Wong H L, Dougherty S, Mccartney-Francis N, Wahl L M, Ellingsworth L, Schmidt J A, Hall G, Roberts A B, Sporn M B. J Immunol. 1988;140:3026–3032. [PubMed] [Google Scholar]
- 4.Musso T, Espinoza-Delgado I, Pulkki K, Gusella G L, Longo D L, Varesio L. Blood. 1990;76:2466–2469. [PubMed] [Google Scholar]
- 5.Warwick-Davies J, Lowrie D B, Cole P J. J Immunol. 1995;155:3186–3193. [PubMed] [Google Scholar]
- 6.Tsunawaki S, Sporn M, Ding A, Nathan C. Nature (London) 1988;334:260–262. doi: 10.1038/334260a0. [DOI] [PubMed] [Google Scholar]
- 7.Ding A, Nathan C F, Graycar J, Derynck R, Stuehr D J, Srimal S. J Immunol. 1990;145:940–944. [PubMed] [Google Scholar]
- 8.Hirsch C S, Yoneda T, Averill L, Ellner J J, Toossi Z. J Infect Dis. 1994;170:1229–1237. doi: 10.1093/infdis/170.5.1229. [DOI] [PubMed] [Google Scholar]
- 9.Toossi Z, Young T G, Averill L E, Hamilton B D, Shiratsuchi H, Ellner J J. Infect Immun. 1995;63:224–228. doi: 10.1128/iai.63.1.224-228.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hirsch C S, Hussain R, Toossi Z, Dawood G, Shahid F, Ellner J J. Proc Natl Acad Sci USA. 1996;93:3193–3198. doi: 10.1073/pnas.93.8.3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dahl K, Shiratsuchi H, Hamilton B D, Ellner J J, Toossi Z. Infect Immun. 1996;64:399–405. doi: 10.1128/iai.64.2.399-405.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Toossi Z, Gogate P, Shiratsuchi H, Young T, Ellner J J. J Immunol. 1995;154:465–473. [PubMed] [Google Scholar]
- 13.Toossi Z, Hirsch C, Hamilton B D, Knuth C K, Friedlander M A, Rich E A. J Immunol. 1996;156:3461–3468. [PubMed] [Google Scholar]
- 14.Schwander S K, Sada E, Torres M, Escobedo D, Sierra J G, Rich E A. J Infect Dis. 1996;173:1267–1272. doi: 10.1093/infdis/173.5.1267. [DOI] [PubMed] [Google Scholar]
- 15.Flynn J, Chan J, Triebold K J, Dalton D K, Steward T A, Bloom B S. J Exp Med. 1993;178:2249–2252. doi: 10.1084/jem.178.6.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cooper A M, Dalton D K, Steward T A, Griffin J P, Russell D G, Orme I A. J Exp Med. 1993;178:2243–2247. doi: 10.1084/jem.178.6.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bermudez L E. J Immunol. 1993;150:1838–1845. [PubMed] [Google Scholar]
- 18.Flaumenhaft R, Kojima S, Abe M, Rifkin D B. Adv Pharmacol. 1993;24:51–76. doi: 10.1016/s1054-3589(08)60933-3. [DOI] [PubMed] [Google Scholar]
- 19.Miyazono K, Heldin C H. Nature (London) 1989;338:158–160. doi: 10.1038/338158a0. [DOI] [PubMed] [Google Scholar]
- 20.Mccartney-Francis N L, Wahl S. J Leukocyte Biol. 1994;55:401–409. doi: 10.1002/jlb.55.3.401. [DOI] [PubMed] [Google Scholar]
- 21.Kresse H, Hausser H, Schoenherr E. Experientia. 1993;49:403–416. doi: 10.1007/BF01923585. [DOI] [PubMed] [Google Scholar]
- 22.Border W A, Noble N A, Yamamoto T, Harper J R, Yamaguchi Y, Pierschbacher M D, Ruoslahti E. Nature (London) 1992;360:361–364. doi: 10.1038/360361a0. [DOI] [PubMed] [Google Scholar]
- 23.Yamaguchi Y, Mann D M, Ruoslahti E. Nature (London) 1990;346:281–284. doi: 10.1038/346281a0. [DOI] [PubMed] [Google Scholar]
- 24.Westergren-Thorsson G, Hernnaes J, Saernstrand B, Oldberg A, Heinegard D, Malmstroem A. J Clin Invest. 1993;92:632–637. doi: 10.1172/JCI116631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.National Tuberculosis and Respiratory Disease Association. Diagnostic Standards and Classification of Tuberculosis. New York: National Tuberculosis and Respiratory Disease Association; 1969. pp. 68–74. [Google Scholar]
- 26.Wallis R S, Amir-Tahmasseb M, Ellner J J. Proc Natl Acad Sci USA. 1990;87:3348–3352. doi: 10.1073/pnas.87.9.3348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barnes P, Mehra V, Rivoire B, Fong S J, Brennan P J, Voegtliner M S, Minden P, Houghten R A, Bloom B S, Modlin R S. J Immunol. 1992;148:1835–1840. [PubMed] [Google Scholar]
- 28.Barnes P, Chatterjee D, Abrams J S, Lu S, Wang E, Yamamura M, Brennan P, Modlin R S. J Immunol. 1992;49:541–547. [PubMed] [Google Scholar]
- 29.Wahl S M. J Clin Immunol. 1992;12:61–74. doi: 10.1007/BF00918135. [DOI] [PubMed] [Google Scholar]
- 30.Chantry D, Turner M, Abney E, Feldmann M. J Immunol. 1989;142:4295–4300. [PubMed] [Google Scholar]
- 31.Espevik T, Figari I S, Shalaby M R, Lackides G A, Lewis G D, Shepard H M, Palladino M A., Jr J Exp Med. 1987;166:571–576. doi: 10.1084/jem.166.2.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kehrl J H, Roberts A B, Wakefield L M, Jakowlew S, Sporn M B, Fauci A. J Immunol. 1986;137:3855–3860. [PubMed] [Google Scholar]
- 33.Rook A H, Kehrl J H, Wakefield L M, Roberts A B, Sporn M B, Burlington D B, Lane H C, Fauci A S. J Immunol. 1986;136:3916–3920. [PubMed] [Google Scholar]
- 34.Espevik T, Figari I S, Ranges G E, Palladino M A. J Immunol. 1988;140:2312–2316. [PubMed] [Google Scholar]
- 35.Su H S, Leite-Morris K A, Braun L, Biron C A. J Immunol. 1991;147:2717–2727. [PubMed] [Google Scholar]
- 36.Czarniecki C W, Chiu H H, Wong G H, Mccabe S M, Palladino M A. J Immunol. 1988;140:4217–4223. [PubMed] [Google Scholar]
- 37.Welch G R, Wong H L, Wahl S M. J Immunol. 1990;144:3444–3448. [PubMed] [Google Scholar]
- 38.Border W A, Ruoslahti E. J Clin Invest. 1992;90:1–7. doi: 10.1172/JCI115821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Silva J S, Twardzik D R, Reed S. J Exp Med. 1991;174:539–544. doi: 10.1084/jem.174.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gazinelli R T, Oswald I P, Hieny S, James S L, Sher A. Eur J Immunol. 1992;22:2501–2506. doi: 10.1002/eji.1830221006. [DOI] [PubMed] [Google Scholar]
- 41.Barral-Netto M, Barral A, Brownell C E, Skeiky Y A W, Ellingsworth L R, Twardzik D R, Reed S G. Science. 1992;257:545–548. doi: 10.1126/science.1636092. [DOI] [PubMed] [Google Scholar]
- 42.Barral A, Barral-Netto M, Yong E C, Brownell C E, Twardzik D R, Reed S G. Proc Natl Acad Sci USA. 1993;90:3442–3446. doi: 10.1073/pnas.90.8.3442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sharma K, Ziyadeh F N. Am J Physiol. 1994;266:F829–F842. doi: 10.1152/ajprenal.1994.266.6.F829. [DOI] [PubMed] [Google Scholar]
- 44.Bernasconi P, Torchiana E, Confalonieri P, Brugoni R, Barresi R, Mora M, Cornelio F, Morandi L, Mantegazza R. J Clin Invest. 1995;96:1137–1144. doi: 10.1172/JCI118101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Khalil N, Bereznay O, Sporn M, Greenberg A H. J Exp Med. 1989;170:727–737. doi: 10.1084/jem.170.3.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Broekelmann T J, Limper A H, Colby T V, Mcdonald J A. Proc Natl Acad Sci USA. 1991;88:6642–6646. doi: 10.1073/pnas.88.15.6642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roman J, Jeon Y-J, Gal A, Perez R L. Am J Med Sci. 1995;309:124–133. doi: 10.1097/00000441-199503000-00002. [DOI] [PubMed] [Google Scholar]
- 48.Pilatte Y, Bignon J, Lambre C R. Biochem Biophys Acta. 1987;923:150–155. doi: 10.1016/0304-4165(87)90138-3. [DOI] [PubMed] [Google Scholar]
- 49.Grotendorst G R, Smale G, Pencev D. J Cell Physiol. 1989;140:396–402. doi: 10.1002/jcp.1041400226. [DOI] [PubMed] [Google Scholar]
- 50.Boettinger E P, Factor V M, Tsang M L-S, Weatherbee J A, Kopp J B, Quan W S, Wakefield L M, Roberts A S, Thorgeirsson S S, Sporn M B. Proc Natl Acad Sci USA. 1996;93:5877–5882. doi: 10.1073/pnas.93.12.5877. [DOI] [PMC free article] [PubMed] [Google Scholar]