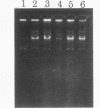
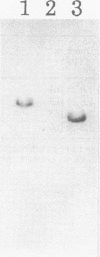
Abstract
The broad-host-range IncP plasmids RP4, R68.45, RP1::Tn501, and pUB307 were transferred to acidophilic, obligately chemolithotrophic Thiobacillus ferrooxidans from Escherichia coli by conjugation. A genetic marker of kanamycin resistance was expressed in T. ferrooxidans. Plasmid RP4 was transferred back to E. coli from T. ferrooxidans. The broad-host-range IncQ vector pJRD215 was mobilized to T. ferrooxidans with the aid of plasmid RP4 integrated in the chromosome of E. coli SM10. pJRD215 was stable, and all genetic markers (kanamycin/neomycin and streptomycin resistance) were expressed in T. ferrooxidans. By the use of suicide vector pSUP1011, transposon Tn5 was introduced into T. ferrooxidans. The influence of some factors on plasmid transfer from E. coli to T. ferrooxidans was investigated. Results showed that the physiological state of donor cells might be important to the mobilization of plasmids. The transfer of plasmids from E. coli to T. ferrooxidans occurred in the absence of energy sources for both donor and recipient.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barros M. E., Rawlings D. E., Woods D. R. Cloning and expression of the Thiobacillus ferrooxidans glutamine synthetase gene in Escherichia coli. J Bacteriol. 1985 Dec;164(3):1386–1389. doi: 10.1128/jb.164.3.1386-1389.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barros M. E., Rawlings D. E., Woods D. R. Production and Regeneration of Thiobacillus ferrooxidans Spheroplasts. Appl Environ Microbiol. 1985 Sep;50(3):721–723. doi: 10.1128/aem.50.3.721-723.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett P. M., Grinsted J., Richmond M. H. Transposition of TnA does not generate deletions. Mol Gen Genet. 1977 Jul 20;154(2):205–211. doi: 10.1007/BF00330839. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta N., Hedges R. W., Shaw E. J., Sykes R. B., Richmond M. H. Properties of an R factor from Pseudomonas aeruginosa. J Bacteriol. 1971 Dec;108(3):1244–1249. doi: 10.1128/jb.108.3.1244-1249.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson M. S., Roy P., Summers A. O. Transpositional Mutagenesis of Thiobacillus novellus and Thiobacillus versutus. Appl Environ Microbiol. 1985 Jun;49(6):1436–1441. doi: 10.1128/aem.49.6.1436-1441.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson M. S., Summers A. O. Wide-host-range plasmids function in the genus thiobacillus. Appl Environ Microbiol. 1983 Sep;46(3):565–572. doi: 10.1128/aem.46.3.565-572.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davison J., Heusterspreute M., Chevalier N., Ha-Thi V., Brunel F. Vectors with restriction site banks. V. pJRD215, a wide-host-range cosmid vector with multiple cloning sites. Gene. 1987;51(2-3):275–280. doi: 10.1016/0378-1119(87)90316-7. [DOI] [PubMed] [Google Scholar]
- Dispirito A. A., Silver M., Voss L., Tuovinen O. H. Flagella and pili of iron-oxidizing thiobacilli isolated from a uranium mine in northern ontario, Canada. Appl Environ Microbiol. 1982 May;43(5):1196–1200. doi: 10.1128/aem.43.5.1196-1200.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Astorga A., Muela A., Cisterna R., Iriberri J., Barcina I. Biotic and abiotic factors affecting plasmid transfer in Escherichia coli strains. Appl Environ Microbiol. 1992 Jan;58(1):392–398. doi: 10.1128/aem.58.1.392-398.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerry P., van Embden J., Falkow S. Molecular nature of two nonconjugative plasmids carrying drug resistance genes. J Bacteriol. 1974 Feb;117(2):619–630. doi: 10.1128/jb.117.2.619-630.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas D., Holloway B. W. R factor variants with enhanced sex factor activity in Pseudomonas aeruginosa. Mol Gen Genet. 1976 Mar 30;144(3):243–251. doi: 10.1007/BF00341722. [DOI] [PubMed] [Google Scholar]
- Jin S. M., Yan W. M., Wang Z. N. Transfer of IncP Plasmids to Extremely Acidophilic Thiobacillus thiooxidans. Appl Environ Microbiol. 1992 Jan;58(1):429–430. doi: 10.1128/aem.58.1.429-430.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulpa C. F., Roskey M. T., Travis M. T. Transfer of plasmid RP1 into chemolithotrophic Thiobacillus neapolitanus. J Bacteriol. 1983 Oct;156(1):434–436. doi: 10.1128/jb.156.1.434-436.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusano T., Sugawara K., Inoue C., Takeshima T., Numata M., Shiratori T. Electrotransformation of Thiobacillus ferrooxidans with plasmids containing a mer determinant. J Bacteriol. 1992 Oct;174(20):6617–6623. doi: 10.1128/jb.174.20.6617-6623.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusano T., Takeshima T., Sugawara K., Inoue C., Shiratori T., Yano T., Fukumori Y., Yamanaka T. Molecular cloning of the gene encoding Thiobacillus ferrooxidans Fe(II) oxidase. High homology of the gene product with HiPIP. J Biol Chem. 1992 Jun 5;267(16):11242–11247. [PubMed] [Google Scholar]
- Mobley H. L., Chen C. M., Silver S., Rosen B. P. Cloning and expression of R-factor mediated arsenate resistance in Escherichia coli. Mol Gen Genet. 1983;191(3):421–426. doi: 10.1007/BF00425757. [DOI] [PubMed] [Google Scholar]
- O'Morchoe S. B., Ogunseitan O., Sayler G. S., Miller R. V. Conjugal transfer of R68.45 and FP5 between Pseudomonas aeruginosa strains in a freshwater environment. Appl Environ Microbiol. 1988 Aug;54(8):1923–1929. doi: 10.1128/aem.54.8.1923-1929.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pretorius I. M., Rawlings D. E., Woods D. R. Identification and cloning of Thiobacillus ferrooxidans structural nif genes in Escherichia coli. Gene. 1986;45(1):59–65. doi: 10.1016/0378-1119(86)90132-0. [DOI] [PubMed] [Google Scholar]
- Rawlings D. E., Pretorius I., Woods D. R. Expression of a Thiobacillus ferrooxidans origin of replication in Escherichia coli. J Bacteriol. 1984 May;158(2):737–738. doi: 10.1128/jb.158.2.737-738.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawlings D. E., Woods D. R. Mobilization of Thiobacillus ferrooxidans plasmids among Escherichia coli strains. Appl Environ Microbiol. 1985 May;49(5):1323–1325. doi: 10.1128/aem.49.5.1323-1325.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richaume A., Angle J. S., Sadowsky M. J. Influence of soil variables on in situ plasmid transfer from Escherichia coli to Rhizobium fredii. Appl Environ Microbiol. 1989 Jul;55(7):1730–1734. doi: 10.1128/aem.55.7.1730-1734.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SILVERMAN M. P., LUNDGREN D. G. Studies on the chemoautotrophic iron bacterium Ferrobacillus ferrooxidans. I. An improved medium and a harvesting procedure for securing high cell yields. J Bacteriol. 1959 May;77(5):642–647. doi: 10.1128/jb.77.5.642-647.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiratori T., Inoue C., Sugawara K., Kusano T., Kitagawa Y. Cloning and expression of Thiobacillus ferrooxidans mercury ion resistance genes in Escherichia coli. J Bacteriol. 1989 Jun;171(6):3458–3464. doi: 10.1128/jb.171.6.3458-3464.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yankofsky S. A., Gurevich R., Grimland N., Stark A. A. Genetic transformation of obligately chemolithotrophic thiobacilli. J Bacteriol. 1983 Feb;153(2):652–657. doi: 10.1128/jb.153.2.652-657.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]