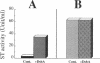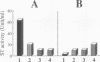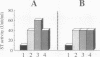Abstract
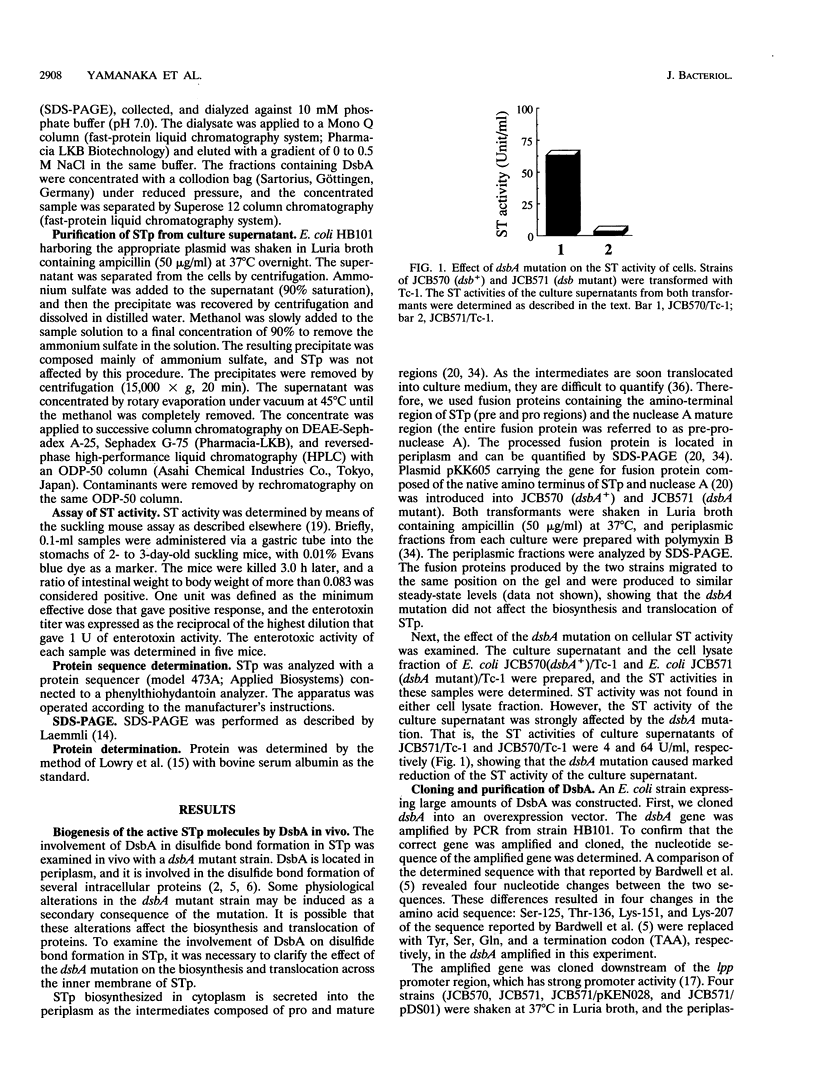
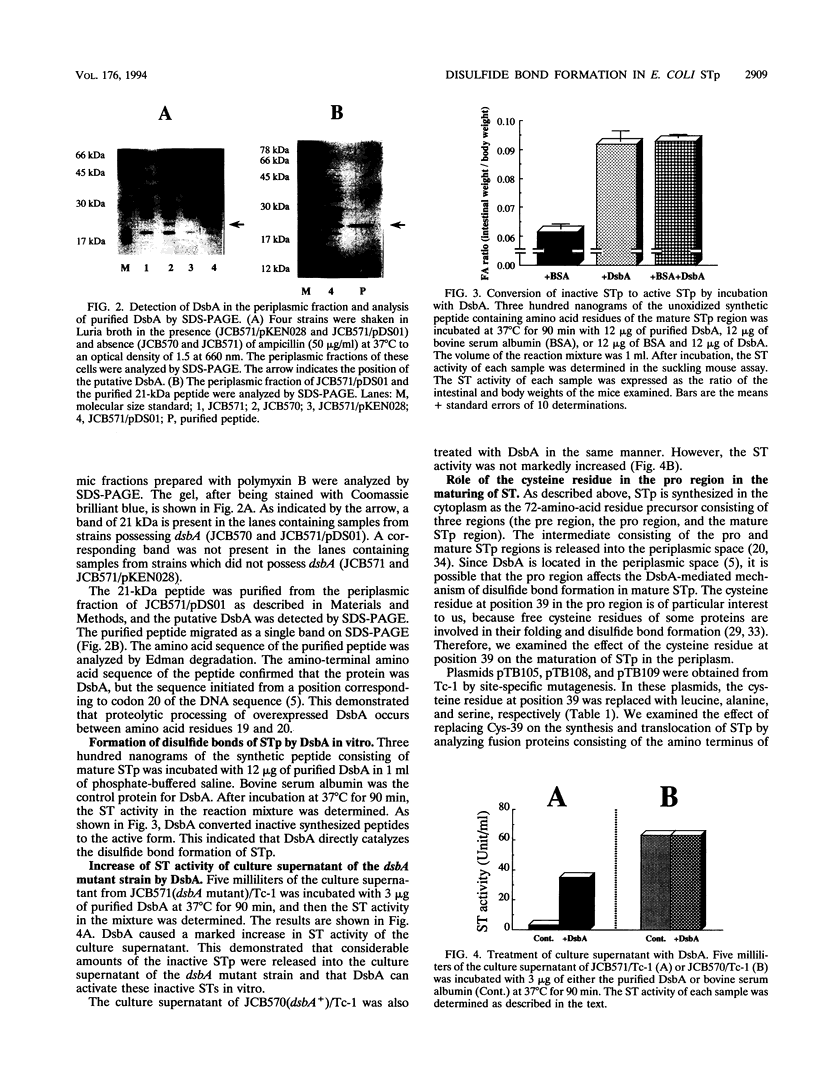
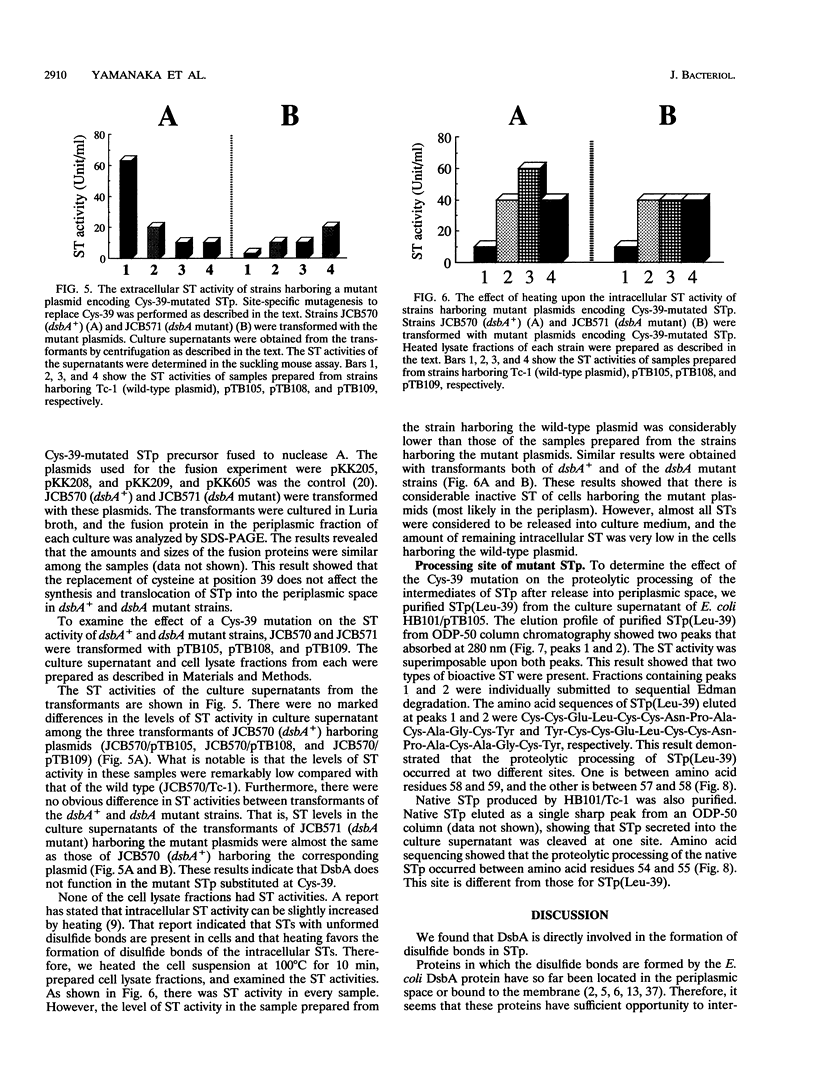
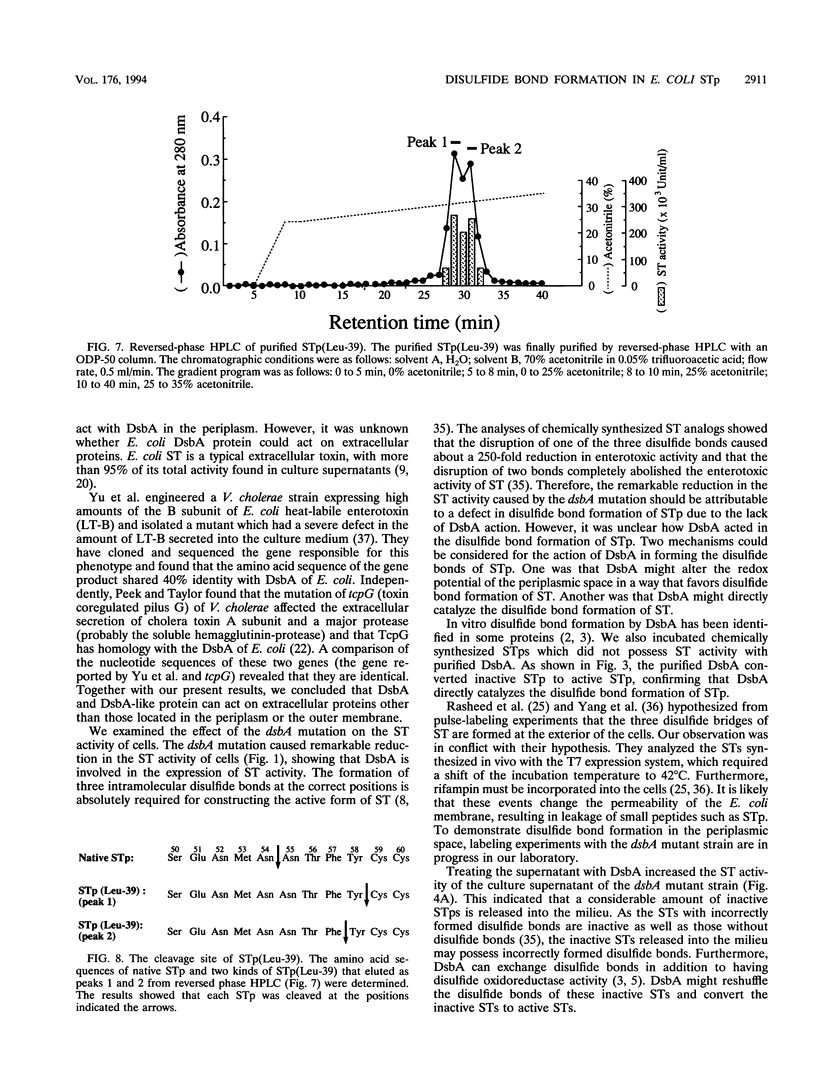
The Escherichia coli heat-stable enterotoxin STp is synthesized as a precursor consisting of pre, pro and mature regions. Mature STp is released into the culture supernatant and is composed of 18-amino-acid resides which contain three intramolecular disulfide bonds. The involvement of DsbA in the formation of the disulfide bonds of STp was examined in this study. A dsbA mutant was transformed with a plasmid harboring the STp gene, and the ST activity was significantly lower than that of the parent strain harboring the same plasmid. Furthermore, purified DsbA induced the conversion of synthetic STp peptide (inactive form) to the active form and increased the ST activity of the culture supernatant derived from the dsbA transformants. These results showed that DsbA directly catalyzes the formation of the disulfide bonds of STp. DsbA is located in periplasmic space, where STp is released as an intermediate form consisting of pro and mature regions. To examine the effect of the pro region on the action of DsbA, we replaced the cysteine residue at position 39 and tested the effect in vivo. The substitution caused a significant decrease of ST activity in the culture supernatant, the accumulation of inactive ST in periplasmic space, and an alteration in the cleavage site of the intermediate of STp. We conclude that Cys-39 is important for recognition by the processing enzymes required for the maturation of STp.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aimoto S., Takao T., Shimonishi Y., Hara S., Takeda T., Takeda Y., Miwatani T. Amino-acid sequence of a heat-stable enterotoxin produced by human enterotoxigenic Escherichia coli. Eur J Biochem. 1982 Dec 15;129(2):257–263. doi: 10.1111/j.1432-1033.1982.tb07047.x. [DOI] [PubMed] [Google Scholar]
- Akiyama Y., Ito K. Folding and assembly of bacterial alkaline phosphatase in vitro and in vivo. J Biol Chem. 1993 Apr 15;268(11):8146–8150. [PubMed] [Google Scholar]
- Akiyama Y., Kamitani S., Kusukawa N., Ito K. In vitro catalysis of oxidative folding of disulfide-bonded proteins by the Escherichia coli dsbA (ppfA) gene product. J Biol Chem. 1992 Nov 5;267(31):22440–22445. [PubMed] [Google Scholar]
- Bardwell J. C., Lee J. O., Jander G., Martin N., Belin D., Beckwith J. A pathway for disulfide bond formation in vivo. Proc Natl Acad Sci U S A. 1993 Feb 1;90(3):1038–1042. doi: 10.1073/pnas.90.3.1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardwell J. C., McGovern K., Beckwith J. Identification of a protein required for disulfide bond formation in vivo. Cell. 1991 Nov 1;67(3):581–589. doi: 10.1016/0092-8674(91)90532-4. [DOI] [PubMed] [Google Scholar]
- Dailey F. E., Berg H. C. Mutants in disulfide bond formation that disrupt flagellar assembly in Escherichia coli. Proc Natl Acad Sci U S A. 1993 Feb 1;90(3):1043–1047. doi: 10.1073/pnas.90.3.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derman A. I., Prinz W. A., Belin D., Beckwith J. Mutations that allow disulfide bond formation in the cytoplasm of Escherichia coli. Science. 1993 Dec 10;262(5140):1744–1747. doi: 10.1126/science.8259521. [DOI] [PubMed] [Google Scholar]
- Greenberg R. N., Dunn J. A., Guerrant R. L. Reduction of the secretory response to Escherichia coli heat-stable enterotoxin by thiol and disulfide compounds. Infect Immun. 1983 Jul;41(1):174–180. doi: 10.1128/iai.41.1.174-180.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman-Verduzco L. M., Kupersztoch Y. M. Rectification of two Escherichia coli heat-stable enterotoxin allele sequences and lack of biological effect of changing the carboxy-terminal tyrosine to histidine. Infect Immun. 1989 Feb;57(2):645–648. doi: 10.1128/iai.57.2.645-648.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzmán-Verduzco L. M., Fonseca R., Kupersztoch-Portnoy Y. M. Thermoactivation of a periplasmic heat-stable enterotoxin of Escherichia coli. J Bacteriol. 1983 Apr;154(1):146–151. doi: 10.1128/jb.154.1.146-151.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamitani S., Akiyama Y., Ito K. Identification and characterization of an Escherichia coli gene required for the formation of correctly folded alkaline phosphatase, a periplasmic enzyme. EMBO J. 1992 Jan;11(1):57–62. doi: 10.1002/j.1460-2075.1992.tb05027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Nakamura K., Inouye M. Construction of versatile expression cloning vehicles using the lipoprotein gene of Escherichia coli. EMBO J. 1982;1(6):771–775. doi: 10.1002/j.1460-2075.1982.tb01244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto K., Okamoto K., Yukitake J., Kawamoto Y., Miyama A. Substitutions of cysteine residues of Escherichia coli heat-stable enterotoxin by oligonucleotide-directed mutagenesis. Infect Immun. 1987 Sep;55(9):2121–2125. doi: 10.1128/iai.55.9.2121-2125.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto K., Okamoto K., Yukitake J., Miyama A. Reduction of enterotoxic activity of Escherichia coli heat-stable enterotoxin by substitution for an asparagine residue. Infect Immun. 1988 Aug;56(8):2144–2148. doi: 10.1128/iai.56.8.2144-2148.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto K., Takahara M. Synthesis of Escherichia coli heat-stable enterotoxin STp as a pre-pro form and role of the pro sequence in secretion. J Bacteriol. 1990 Sep;172(9):5260–5265. doi: 10.1128/jb.172.9.5260-5265.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaki H., Sato T., Kubota H., Hata Y., Katsube Y., Shimonishi Y. Molecular structure of the toxin domain of heat-stable enterotoxin produced by a pathogenic strain of Escherichia coli. A putative binding site for a binding protein on rat intestinal epithelial cell membranes. J Biol Chem. 1991 Mar 25;266(9):5934–5941. [PubMed] [Google Scholar]
- Peek J. A., Taylor R. K. Characterization of a periplasmic thiol:disulfide interchange protein required for the functional maturation of secreted virulence factors of Vibrio cholerae. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):6210–6214. doi: 10.1073/pnas.89.13.6210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugsley A. P., Kornacker M. G., Poquet I. The general protein-export pathway is directly required for extracellular pullulanase secretion in Escherichia coli K12. Mol Microbiol. 1991 Feb;5(2):343–352. doi: 10.1111/j.1365-2958.1991.tb02115.x. [DOI] [PubMed] [Google Scholar]
- Pugsley A. P. Translocation of a folded protein across the outer membrane in Escherichia coli. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):12058–12062. doi: 10.1073/pnas.89.24.12058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasheed J. K., Guzmán-Verduzco L. M., Kupersztoch Y. M. Two precursors of the heat-stable enterotoxin of Escherichia coli: evidence of extracellular processing. Mol Microbiol. 1990 Feb;4(2):265–273. doi: 10.1111/j.1365-2958.1990.tb00593.x. [DOI] [PubMed] [Google Scholar]
- Shimonishi Y., Hidaka Y., Koizumi M., Hane M., Aimoto S., Takeda T., Miwatani T., Takeda Y. Mode of disulfide bond formation of a heat-stable enterotoxin (STh) produced by a human strain of enterotoxigenic Escherichia coli. FEBS Lett. 1987 May 4;215(1):165–170. doi: 10.1016/0014-5793(87)80134-5. [DOI] [PubMed] [Google Scholar]
- So M., McCarthy B. J. Nucleotide sequence of the bacterial transposon Tn1681 encoding a heat-stable (ST) toxin and its identification in enterotoxigenic Escherichia coli strains. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4011–4015. doi: 10.1073/pnas.77.7.4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staples S. J., Asher S. E., Giannella R. A. Purification and characterization of heat-stable enterotoxin produced by a strain of E. coli pathogenic for man. J Biol Chem. 1980 May 25;255(10):4716–4721. [PubMed] [Google Scholar]
- Steiner D. F., Clark J. L. The spontaneous reoxidation of reduced beef and rat proinsulins. Proc Natl Acad Sci U S A. 1968 Jun;60(2):622–629. doi: 10.1073/pnas.60.2.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahara M., Hibler D. W., Barr P. J., Gerlt J. A., Inouye M. The ompA signal peptide directed secretion of Staphylococcal nuclease A by Escherichia coli. J Biol Chem. 1985 Mar 10;260(5):2670–2674. [PubMed] [Google Scholar]
- Takao T., Hitouji T., Aimoto S., Shimonishi Y., Hara S., Takeda T., Takeda Y., Miwatani T. Amino acid sequence of a heat-stable enterotoxin isolated from enterotoxigenic Escherichia coli strain 18D. FEBS Lett. 1983 Feb 7;152(1):1–5. doi: 10.1016/0014-5793(83)80469-4. [DOI] [PubMed] [Google Scholar]
- Tomb J. F. A periplasmic protein disulfide oxidoreductase is required for transformation of Haemophilus influenzae Rd. Proc Natl Acad Sci U S A. 1992 Nov 1;89(21):10252–10256. doi: 10.1073/pnas.89.21.10252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman J. S., Kim P. S. The pro region of BPTI facilitates folding. Cell. 1992 Nov 27;71(5):841–851. doi: 10.1016/0092-8674(92)90559-u. [DOI] [PubMed] [Google Scholar]
- Yamanaka H., Fuke Y., Hitotsubashi S., Fujii Y., Okamoto K. Functional properties of pro region of Escherichia coli heat-stable enterotoxin. Microbiol Immunol. 1993;37(3):195–205. doi: 10.1111/j.1348-0421.1993.tb03200.x. [DOI] [PubMed] [Google Scholar]
- Yang Y., Gao Z., Guzmán-Verduzco L. M., Tachias K., Kupersztoch Y. M. Secretion of the STA3 heat-stable enterotoxin of Escherichia coli: extracellular delivery of Pro-STA is accomplished by either Pro or STA. Mol Microbiol. 1992 Dec;6(23):3521–3529. doi: 10.1111/j.1365-2958.1992.tb01787.x. [DOI] [PubMed] [Google Scholar]
- Yu J., Webb H., Hirst T. R. A homologue of the Escherichia coli DsbA protein involved in disulphide bond formation is required for enterotoxin biogenesis in Vibrio cholerae. Mol Microbiol. 1992 Jul;6(14):1949–1958. doi: 10.1111/j.1365-2958.1992.tb01368.x. [DOI] [PubMed] [Google Scholar]
- Zapun A., Bardwell J. C., Creighton T. E. The reactive and destabilizing disulfide bond of DsbA, a protein required for protein disulfide bond formation in vivo. Biochemistry. 1993 May 18;32(19):5083–5092. doi: 10.1021/bi00070a016. [DOI] [PubMed] [Google Scholar]