Abstract
Infection with the protozoan parasite Trypanosoma cruzi often results in chronic heart- and gut-associated disease known as Chagas disease. In this study we show that contrary to previous reports, neonatal hearts transplanted into mice chronically infected with T. cruzi do not exhibit signs of autoimmune-type rejection or any significant inflammatory response. In addition to an absence of inflammation, these syngeneic heart transplants survive for more than 1 year and are absolutely free of parasites as determined by in situ PCR analysis. However, if well-established transplanted hearts in chronically infected mice are directly injected with live parasites, a rapid and dramatic inflammatory response ensues that results in cessation of heart function. Likewise, transplanted hearts established in mice prior to systemic infection with T. cruzi or hearts transplanted into mice during the acute stage of T. cruzi infection become parasitized and develop inflammatory foci. In these cases where the transplanted hearts become parasitized, the ensuing inflammatory response is nearly identical to that observed in the native hearts of T. cruzi-infected mice in terms of cell types present and adhesion molecules and cytokines expressed. Importantly, this response is strikingly different from that observed in the allogeneic heart rejection. These results clearly document that parasitization of heart tissue is both necessary and sufficient for the induction of tissue damage in Chagas disease and strongly argue against a principal autoimmune etiology for this disease.
Trypanosoma cruzi infects up to 18 million people in Latin American, and 90 million individuals are at risk of infection. Most of the mortality resulting from T. cruzi infection is a consequence of heart failure during the chronic phase of the disease rather than due to complications of the acute infection. Chagas disease is thought to be the single most common cause of congestive heart failure and sudden death in the world and the leading cause of death among young-to-middle age adults in endemic areas of Latin America.
Despite its medical importance, the etiology of Chagas disease is not fully understood. A number of mechanisms have been proposed to account for chronic chagasic pathology including direct parasite-mediated tissue destruction, loss of nervous tissue function, intravascular platelet aggregation, and generation of autoimmune reactivity (reviewed in refs. 1 and 2). The scarcity of parasites in chronic inflammatory lesions, the presence of polyclonal activation in the acute infection (3), and the existence of “shared epitopes” between the parasite and host heart and neuronal tissue (4–6) have all been used to support an argument for an autoimmune mechanism for Chagas disease.
Although autoimmunity is still considered by many to be the likely cause of Chagas disease, the evidence in support of this hypothesis is still largely circumstantial. While the presence of “anti-self” immune responses has been unquestionably demonstrated, their participation in disease development has not been. Probably the most convincing evidence in support of an autoimmunity in development of Chagas disease comes from studies using a heterotopic (ear) neonatal heart transplant model. Ribeiro-dos-Santos et al. (7) showed that syngeneic neonatal hearts transplanted into chronically infected mice were rapidly rejected in a time course similar to that of allografted hearts.
We were interested in utilizing this heart transplant model to further characterize the immunological events involved in chronic chagasic heart disease. In this work we demonstrate that heart “rejection” in chronic T. cruzi infection depends upon the presence of parasites in the heart tissue; in the absence of local parasitization, no inflammatory reaction is observed and consequently no “rejection” occurs.
MATERIALS AND METHODS
Mice and Parasites.
Female C57BL/6J or male C3H/HeSnJ mice (6–8 weeks of age) were infected intraperitoneally with 103 blood-form trypomastigotes of the Brazil strain of T. cruzi, 106 tissue-culture-derived trypomastigotes of the Sylvio X10/4 clone, or 103 tissue-culture-derived trypomastigotes of the Y strain.
Heart Transplants.
Infected mice, usually between 180 and 400 days postinfection, and age-matched, uninfected controls were used as recipients of heart transplants. These recipients were anesthetized with rompun (3.5 mg/kg) and ketamine (35 mg/kg) and a subcutaneous pocket on the dorsum of one ear was made by incising the skin over the auricular artery at the base of the ear. Donor hearts from fetal (day 18–21 gestation) or neonatal (less that 2 days old) mice were briefly placed in normal saline and then eased into the pocket near the distal edge of the ear. Gentle pressure was used to remove trapped air. Observations of the grafts were made using ×16 magnification and translumination. Beating of the hearts could generally be detected within 7 days after grafting. Electrocardiogram (ECG) monitoring of the transplanted heart was also used to obtain an earlier and more accurate determination of the acceptance of the grafted heart (8).
Histochemistry and Immunocytochemistry of Transplanted Tissues.
Transplanted hearts as well as the native heart in transplant recipients were either processed by formalin fixation/parafin embedding followed by standard hematoxylin/eosin staining, or were frozen for immunocytochemical analysis as described (9, 10). Native or transplanted hearts were analyzed for the presence of inflammation, cell surface markers, and cytokine-producing cells. Inflammatory scores were derived as described (10). Scores for CD4+, CD8+, or cytokine-producing cells were obtained by counting the total number of positively stained cells in five noncontiguous ×25 microscopic fields: −, no positive cells; +, 1–10 positive cells; ++, 11–25 positive cells; and +++, >25 positive cells. For major histocompatibility complex (MHC) and adhesion molecules the following grading scale was used: −, not detectable; +, foci of positive staining in <10% of the heart section examined; ++, positive staining in 10–20% of the heart section; +++, positive staining in >20% of the section.
In Situ PCR Detection of T. cruzi Kinetoplast DNA (kDNA).
The presence of T. cruzi in heart tissue was assessed by in situ PCR analysis (11) using T. cruzi kDNA-specific oligonucleotides. Native and transplanted hearts were fixed for 5 hr in 10% neutral buffered formaldehyde followed by four washes (30 min each) in PBS. The tissue was then snap-frozen in liquid NO2 and 6- to 10-μm-thick cryosections were cut and air-dried on microscope slides. The sections were further fixed in 90% acetone (5 min) and 5% buffered formaldehyde (5 min), washed in PBS (four washes of 30 min each), and blocked in 2% casein solution (30 min) prior to storage at −80°C. The PCR was carried out in a GeneAmp In Situ PCR System 1000 (Perkin–Elmer) according to manufacturer’s instructions. Primers matching the conserved regions of T. cruzi kDNA (12) were used to amplify the kDNA on tissue sections. The forward primer (5′-GGTTCGATTGGGGTTGGTGTAATATA-3′) and reverse, biotin-labeled primer (5′-biotin-CCAAAATTTGAACGCCCCTCCCAAAA-3′) were used at 0.1 μM in PCR buffer (10 mM Tris·HCl/50 mM KCl, pH 8.3; Boehringer Mannheim) containing 1.5 mM Mg2+, 0.5 mM dNTP, and 25 units/ml Taq polymerase. Following an initial incubation at 95°C for 5 min, 60°C for 2 min, and 72°C for 2 min, the reaction was cycled 29 times using the following settings: 95°C for 10 sec, 60°C for 30 sec, and 72°C for 1 min. The slides were then washed in PBS (four washes for 20 min each), 0.05% Tween 20 in PBS (three washes for 10 min each), PBS (four washes for 15 min each), and incubated in avidin-peroxidase (Vector Laboratories) for 45 min at room temperature. Finally, the sections were washed in PBS-Tween 20 and in PBS alone again, as above, before the peroxidase was detected with 3′,3′-diaminobenzidine tetrahydrochloride (Sigma) and the sections counter-stained with hematoxylin.
RESULTS
C3H(He)SnJ mice infected with the Sylvio X10/4 clone of T. cruzi exhibit extensive heart inflammation and tissue damage throughout the acute and chronic phases of the infection (13). To understand better the progression of disease in this model of human Chagas disease, we conducted heterotopic transplantation of neonatal hearts into these mice as described (7). Age-matched noninfected mice were also used as transplant recipients. Transplanted hearts were observed for the presence of mechanical activity (heart beat), electrical activity (ECG), and histologically for the presence of inflammation and tissue damage.
In multiple experiments, both control and T. cruzi-infected mice accepted the neonatal heart transplants in approximately the same proportion with only an occasional failure (9% and 13% failure rate in normal and infected mice, respectively; Table 1). These failures were either due to mechanical damage to the heart during excision from the donor or to apparent secondary bacterial infections at the site of the transplant. Beating of transplanted hearts in either normal or infected mice was usually obvious within 7 days posttransplantation and once so established these hearts continued to beat and to appear healthy when observed up to 1 year posttransplantation.
Table 1.
Survival of heterotopic neonatal heart transplants in T. cruzi-infected mice
| Recipient strain | Parasite strain | D.P.I. | Failures/ total | |
|---|---|---|---|---|
| Exp. 1 | C3H(He)SnJ | Sylvio X10/4 | 180–380 | 2/18 |
| C3H(He)SnJ | None | 1/8 | ||
| Exp. 2 | C3H(He)SnJ | Sylvio X10/4 | 210–230 | 2/13 |
| C3H(He)SnJ | None | 1/14 |
Syngeneic neonatal hearts were transplanted into age-matched C3H(He)SnJ male mice that were either not infected or were infected 180–380 days previously (D.P.I.) with the Sylvio X10/4 clone of T. cruzi. The number of hearts that failed to beat within 14 days posttransplantation from the total transplanted is indicated.
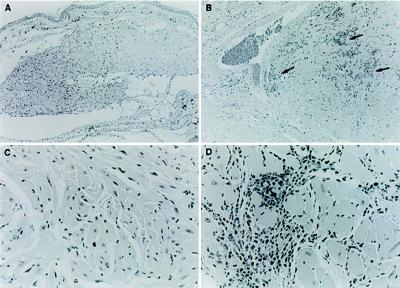
Histological analysis of hearts transplanted into chronically infected mice revealed no evidence of an inflammatory response in the transplanted tissue (Fig. 1). The tissue in the transplanted hearts, particularly in hearts that have been in the recipient for more than 1–2 months, is generally less well organized than in the native heart. This effect has been previously ascribed to the lack of hemodynamic load on the transplanted heart (14). However, the transplanted hearts lacked the characteristic inflammatory foci and tissue destruction evident in the native chagasic heart (Fig. 1). The failure of T. cruzi-infected mice to reject syngeneic heart transplants was not a peculiarity of a particular mouse/parasite strain combination: C57BL/6J mice infected with either the Brazil or Y strain of T. cruzi also failed to reject syngeneic neonatal heart transplants (data not shown).
Figure 1.
Neonatal hearts establish and survive for greater than 6 months in mice with chronic (>200 days) T. cruzi infections. The transplanted heart tissue (A and C), although appearing less organized in comparison to the native heart of the same mouse (B and D), nevertheless lacks any evidence of the intense inflammatory response obvious in the native heart (arrows). (A and B, ×63; C and D, ×250.)
In contrast to the failure of chronically infected mice to reject transplanted hearts, hearts transplanted into mice on the day of infection (day 0) or into mice at the midpoint of the acute infection (day 30) were rejected (4 of 4 mice). These hearts became parasitized and exhibited similar signs of inflammation as the native heart of these same animals (Table 2). This result demonstrates that the transplanted hearts are accessible to both circulating parasites and to the inflammatory cells which normally comprise the heart infiltrates in Chagas disease.
Table 2.
Inflammatory response in native and transplanted hearts
| Group | n | I.S. | CD8 | CD4 | TNF-α | TGF-β | IFN-γ | IL-1 | IL-2 | IL-6 | MHC I | MHC II | ICAM-1 | VCAM-1 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Normal | ||||||||||||||
| Native | 6 | − | − | − | − | − | − | − | − | − | − | − | +/− | − |
| Transplanted | 6 | − | − | − | − | − | − | − | − | − | − | − | +/− | − |
| Acute infection | ||||||||||||||
| Native | 4 | ++ | ++ | + | + | + | − | − | − | − | ND | ND | ND | ND |
| Transplanted | 4 | ++ | ++ | + | +/++ | +/++ | − | +/− | ND | − | ND | ND | ND | ND |
| Chronic infection | ||||||||||||||
| Native | 19 | +++ | ++/+++ | + | ++ | ++ | − | +/++ | − | +/++ | ++/+++ | ++/ | ++/+++ | +/++ |
| +++ | ||||||||||||||
| Transplanted | 5 | − | − | − | − | − | − | − | − | − | + | + | + | + |
| Transplanted plus PBS | 4 | − | − | − | − | − | − | − | − | − | + | + | + | + |
| Transplanted plus parasites | 10 | ++/+++ | ++/+++ | + | ++ | ++ | − | +/++ | − | +/++ | + | +/++ | +/++ | + |
| Transplanted plus killed parasites | 3 | +/++ | + | + | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| Allotransplanted | 2 | +++ | ++ | ++/++ | ++ | ++ | + | ++ | + | + | ++ | ++ | ND | ++ |
Scores for the degree of inflammation (I.S.) and for cell surface markers and cytokine-producing cells were obtained as described. Transplanted hearts in normal or chronically infected (>200 days post-infection) mice were examined 20–60 days posttransplantation. Injections of PBS or heat-killed parasites (1 million in 10 μl) were made into hearts 2–14 weeks posttransplantation and examined 10–50 days later. Transplants into acutely infected mice were done at day 24 post-infection and examined 15 days later. Allografted hearts were examined 14 days posttransplantation. n, number of hearts examined; ND, not determined; TNF-α, tumor necrosis factor α; TGF-β, transforming growth factor-β; IFN-γ, interferon γ; IL, interleukin; ICAM-1, intercellular adhesion molecule 1; VCAM-1, vascular adhesion molecule 1.
Because the level of circulating parasites is significantly higher in the acute stage of T. cruzi infection than in the chronic stage, the destruction of transplanted heart tissue in acutely infected mice might be a result of the higher level of parasitization of the transplanted hearts in these mice relative to that in mice with chronic T. cruzi infection. This suggestion was confirmed by the demonstration that injection of 106 live parasites into established transplanted hearts in chronically infected mice resulted in rapid and explosive inflammation and cessation of heart function (Table 3 and Fig. 2). As a result of this massive inflammatory response, transplanted hearts injected with live parasites suffered uniform electrical and mechanical failure in all of the 21 mice tested. Injection of PBS as a control had no effect on heart function.
Table 3.
Injection of live but not killed parasites results in loss of electrical and mechanical activity in transplanted hearts
| Group | D.P.I. | Treatment | ECG | Heart failure |
|---|---|---|---|---|
| 1 | Not infected | PBS | ND | 0/4 |
| 2 | Not infected | Live T. cruzi | ND | 8/8 |
| 3 | 315–338 | PBS | ND | 0/4* |
| 4 | 315–338 | Live T. cruzi | ND | 6/6 |
| 5 | 131 | Live T. cruzi | 0/4 positive | 4/4 |
| 6 | 530 | Live T. cruzi | 0/8 positive | 8/8 |
| 7 | 265 | Live T. cruzi | 0/3 positive | 3/3 |
| 8 | 265 | Dead T. cruzi | 2/3 positive | 1/3 |
Transplanted hearts were allowed to establish in mice for 14–21 days and were then injected with 10 μl of PBS or 1 million live trypomastigotes of T. cruzi or the same number of heat-killed (100°C, 5 min) trypomastigotes in 10 μl of PBS. Hearts were observed for ECG activity prior to injection and at 14–21 days postinjection (DPI). Heart failure was determined by visual examination of beating on days 6 and 12 postinjection. ND, not determined. *The heart in one mouse in group 3 stopped beating immediately after injection of PBS but showed no signs of inflammation.
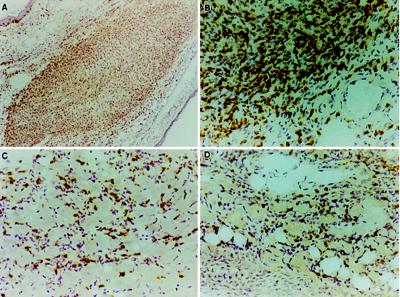
Figure 2.
Injection of parasites into an established transplant results in a massive infiltration of CD8+ T cells. Established heart transplant 14 days after injection of parasites has virtually all of the muscle tissue displaced by inflammatory cells that stain positively (yellow/brown precipitate) for the CD8 molecule (A and B). The native heart of the same animal also exhibits a CD8+ cell-dominated inflammation (C). The parasite-injected transplanted heart contains a large number of cells which are producing tumor necrosis factor α (D). (A, ×63; B–D, ×250.)
Importantly, the characteristics of the inflammatory response, including the types of infiltrating cells and the expression of cytokines, MHC, and adhesion molecules were similar in the native hearts and the transplanted hearts injected with parasites (Table 2 and Fig. 2). CD8+ T cells were by far the predominant inflammatory cell population in both sites and were accompanied by high level expression of tumor necrosis factor α, transforming growth factor β, and interleukin 1 as we have previously described for the native hearts of T. cruzi infected mice (10). Up-regulation of both class I and class II MHC expression and vascular adhesion molecule 1 expression was also evident in the native and parasite-injected transplanted heart. Interestingly, increased MHC and adhesion molecule expression was also present in transplanted hearts that did not receive an injection of parasites. Nevertheless, infiltrating lymphocytes were totally absent in these hearts. The pattern of inflammatory cells and cytokine production in parasite-injected transplanted hearts is in stark contrast to that observed in allogeneic heart transplants. In the latter case, CD4+ T cell predominate and significant numbers of interferon-γ and interleukin 2-producing cells are evident (Table 2).
Although injection of 106 heat-killed parasites into established heart transplants induced a moderate inflammatory response, this nonviable stimulus resulted in loss of heart function in only one of three animals (Tables 2 and 3). As in chronically infected mice, injection of live parasites into hearts transplanted into noninfected recipients resulted in loss of heart function (Table 3). Thus, local infection with T. cruzi was both necessary and sufficient for the consistent development of disease in syngeneic neonatal heart transplants in mice. Injection of lower doses of parasites into transplanted hearts in chronically infected mice established that a threshold level of parasites is required to generate the response necessary to cause loss of function in the transplanted hearts; injection of fewer than 500 parasites did not affect the electrical function of established heart transplants (6 of 6 mice); however, injection of 5,000 parasites or greater resulted in the loss of ECG activity in all mice (n = 9) by day 21 postinjection (data not shown).
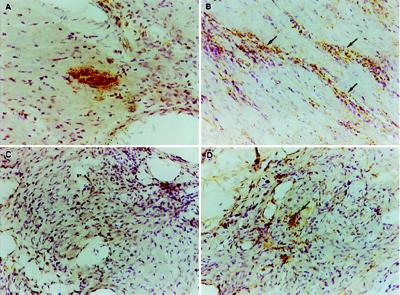
Transplanted hearts, however, are presumably subjected to a constant exposure to circulating parasites in the chronically infected mouse. Nevertheless these transplanted hearts show no sign of infection, even when analyzed by in situ PCR (Fig. 3). This procedure readily detects T. cruzi-infected cells in acutely infected hearts as well as the apparent remnants of parasites in the hearts of chronically infected mice in association with inflammatory foci. However hearts transplanted into chronically infected mice up to 7 months previously show absolutely no evidence of parasitization unless the hearts are directly injected with T. cruzi (Fig. 3). Thus in this chronically infected mouse model, parasites are apparently restricted from spreading to heart tissue in the ear and as a result, this tissue fails to exhibit the classical signs of Chagas disease.
Figure 3.
In situ PCR detection of parasite kDNA in native and transplanted hearts. Detection of a parasite-infected cell (pseudocyst) in the heart of a mouse 14 days postinfection (A) demonstrates the specificity of the in situ PCR for the parasite. Native heart tissue from chronically infected mice (350 days) shows no intact parasite-infected cells but displays a diffuse staining pattern that is more intense in areas of heavy inflammation (arrows in B). The transplanted heart present for 200 days in the same animal as in B shows no evidence of either intact or diffusely distributed parasite kDNA, nor any evidence of inflammation (C). Five days after injection of 106 parasites into a transplanted heart, parasite kDNA is readily detected by in situ PCR (D). The detection of kDNA in the parasite-injected transplanted heart precedes the inflammatory response evident by day 15 postinjection (see Fig. 2). (×250.)
DISCUSSION
The etiology of Chagas disease has been the focus of much investigation, discussion, and controversy. Clinical evidence of Chagas disease occurs in less than half of all individuals infected with T. cruzi and is characterized by primarily mononuclear infiltrates and fibrosis in the heart, and with less frequency, the gut. The slow increase in disease severity in the apparent absence of significant numbers of parasites in the target organs is the major justification for classifying Chagas disease as an autoimmune disorder. An autoimmune etiology accounts for many of the characteristics of the disease state, including the spectral nature of the disease, the organ specificity, the age-related onset, and the presence of anti-self immune responses (reviewed in ref. 15). Discovery and characterization of putative autoantigens that share epitopes with parasite molecules has strengthened the autoimmune hypothesis (4–6). However, despite this wealth of data demonstrating the presence of anti-self responses in T. cruzi infection and Chagas disease, there has been no firm link established between these autoreactivities and the severity of disease in infected hosts or the induction of disease (by cell or serum transfer) in noninfected hosts.
Perhaps the most compelling evidence in favor of an autoimmune etiology for Chagas disease comes from the demonstration that mice with chronic T. cruzi infection vigorously reject normal syngeneic heart transplants (7). This evidence strongly suggested that T. cruzi infection induced anti-heart T cell responses capable of destroying normal heart tissue and that such responses could account for the tissue destruction in the native heart. The major effector cell in this response appeared to be the CD4+ T cell since depletion of this T cell subset could prevent the rejection phenomenon. Because we and others had previously shown that CD8+ T cells are normally the major constituent of the heart infiltrate in chagasic hearts (16–19), we wished to explore this heart transplant model as a means of determining the role of these cells, as well as cells producing cytokines that are prevalent in the native heart (10), in heart tissue damage.
The results of our investigation firmly establish that normal (nonparasitized) heart tissue is not the target of inflammatory responses in chronic murine T. cruzi infection. Mice of two different strains chronically infected with three different isolates of T. cruzi fail to generate even a moderate inflammatory response to heterotopic syngeneic heart transplants. This is the case even when the hearts are observed up to 1 year after transplantation. Hearts transplanted into chronically infected mice express elevated levels of class I and class II MHC molecules and intercellular adhesion molecule 1 and vascular adhesion molecule 1. These molecules are presumably up-regulated by the high systemic level of cytokines such as interferon-γ (9, 20, 21) and tumor necrosis factor α (22, 23) since in situ production of these cytokines was not detected in the transplanted tissue. However the increase in MHC and adhesion molecule expression is not sufficient for the recruitment of inflammatory cells into the transplant as previously suggested (24). The only conditions under which inflammation in and “rejection” of the transplanted heart is observed are (i) transplantation of hearts into mice during the acute phase of the infection, or (ii) direct injection of relatively large numbers of parasites into the transplanted tissue. In these cases, the transplanted heart takes on all the appearances of the native hearts in these animals, including the predominance of CD8+ T cells in the cellular infiltrate, and the high in situ production of tumor necrosis factor α, transforming growth factor β, and interleukin 1. Injection of 106 killed parasites also induced moderate inflammation and cessation of electrical and mechanical activity in only one of three hearts tested. Although more extensive studies would be needed to to make firm conclusions concerning the effects of killed parasites, it is clear that killed parasites do not elicit an inflammatory response similar to that observed in either native hearts or parasite-infected transplanted hearts in chronically infected mice, or the consistent deleterious effects on heart function that live parasites do (i.e., 30 of 30 hearts cease function with as few as 5,000 injected parasites). These results thus provide convincing evidence that local infection of heart tissue is required for the induction of the pathogenic responses characteristic of chronic Chagas disease.
The difference between the results reported herein and those published previously showing rejection of syngeneic transplants in T. cruzi-infected mice likely reflects the characteristics of the infection in the models used. The previous studies used parasite/mouse strain combinations that result in high parasitemic infections and host death during the acute phase unless the mice are rescued by treatment with anti-parasitic drugs (7). It is possible that the higher load of circulating parasites in these mice leads to a more efficient infection of the transplanted tissue and its resulting destruction. We specifically chose to focus this study on the C3H-Sylvio X10/4 infection model of chronic chagasic heart disease. This mouse/parasite strain combination very accurately simulates the human infection, with nearly undetectable parasitemia during the acute and chronic phases of the infection, the absence of mortality in the acute infection, and the development in the heart of inflammation and tissue damage with characteristics remarkably similar to that observed in chagasic human hearts (25, 26).
The absence of parasites in heart tissue even 8 months after its implantation into the chronically infected mice used in this study is very surprising. It was assumed that the release of trypomastigotes from infected host cells would provide an ample source for parasitization of the implanted muscle. It is clear, however, from the results of in situ PCR analysis that while parasites (or at least parasite kDNA) persist in the native hearts years after the initial infection, they are totally absent in the transplanted tissue. The failure of the transplanted tissue to become parasitized demonstrates the efficiency of the anti-parasite immune response in controlling the spread of parasites to other sites in the body, a finding that is supported by the difficulty in detecting circulating parasites in chronically infected hosts (27) as well as previous studies documenting the restriction of tissue parasites to the heart and skeletal muscle in C3H mice chronically infected with the Sylvio X/10 clone of T. cruzi (25). Despite the efficiency of the anti-parasite immune response in infected hosts, there is little evidence that this response can rid the native heart tissue of its parasite burden.
In the native hearts of chronically infected mice, the kDNA PCR product is closely associated with inflammatory infiltrates. This finding as well as the detection of parasites and parasite kDNA in the parasite-injected transplanted heart preceding the inflammatory process provides compelling evidence of a causal link between the presence of parasites in tissues and the inflammatory disease process. The proposed link between parasite load and disease severity in T. cruzi infection is also supported by a growing body of evidence connecting disease severity with the virulence of the infecting parasites (28, 29), the persistence of parasites in the diseased tissues (30–33), and the efficacy of anti-parasite chemotherapy (34–36).
The results of these studies have considerable implications for the treatment or prevention of Chagas disease. Most importantly these data provide theoretical support for efforts to reduce the parasite load in patients as a means of minimizing the severity of Chagas disease. The worry of exacerbation of disease has been the major impediment to the exploration of anti-T. cruzi vaccines. Likewise the development of better anti-parasite chemotherapies and their use in treating chronic as well as acute cases of T. cruzi infection may now be considered in a more favorable light. From a practical point of view vaccine-induced immunity and chemotherapy would appear to hold greater promise for prevention and treatment of Chagas disease than would immunosuppressive therapies that are commonly used to treat autoimmune diseases. This point is emphasized by the fact that essentially any suppression of the immune system during the acute or chronic stages of the infection results not in alleviation but in exacerbation of the disease (19, 37–40). Thus it seems that even if Chagas disease has an autoimmune component, it is unlikely that it can be treated as a conventional autoimmune disease.
Acknowledgments
We thank Dr. Miriam Postan for helpful discussions and for help with the histopathological analysis and Mr. Mark Heiges and Ms. Bolyn Hubby for excellent technical assistance. This work was supported by Public Health Service Grants AI-22070 and AI-33106 from the National Institutes of Health. R.L.T. is a Burroughs Wellcome Fund Scholar in Molecular Parasitology.
ABBREVIATIONS
- ECG
electrocardiogram
- MHC
major histocompatibility complex
- kDNA
kinetoplast DNA
References
- 1.Tanowitz H B, Kirchhoff L V, Simon D, Morris S A, Weiss L M, Wittner M. Clin Microbiol Rev. 1992;5:400–419. doi: 10.1128/cmr.5.4.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rossi M A, Bestetti R B. Cardiology. 1995;86:1–7. doi: 10.1159/000176822. [DOI] [PubMed] [Google Scholar]
- 3.Minoprio P, Itohara S, Heusser C, Tonegawa S, Coutinho A. Immunol Rev. 1989;112:183–207. doi: 10.1111/j.1600-065x.1989.tb00558.x. [DOI] [PubMed] [Google Scholar]
- 4.Rizzo L V, Cunha-Neto E, Teixeira A R L. Infect Immun. 1989;57:2640–2644. doi: 10.1128/iai.57.9.2640-2644.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van Voorhis W C, Schlekewy L, Trong H L. Proc Natl Acad Sci USA. 1991;88:5993–5997. doi: 10.1073/pnas.88.14.5993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cunha-Neto E, Duranti M, Gruber A, Zingales B, De Messias I, Stolf N, Bellotti G, Patarroyo M E, Pilleggi F, Kalil J. Proc Natl Acad Sci USA. 1995;92:3541–3545. doi: 10.1073/pnas.92.8.3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ribeiro-dos-Santos R, Rossi M A, Laus J L, Silva J S, Savino W, Mengel J. J Exp Med. 1992;175:29–39. doi: 10.1084/jem.175.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fulmer R J, Cramer A T, Liebelt R A, Liebelt A G. Am J Anat. 1963;113:273–285. doi: 10.1002/aja.1001130206. [DOI] [PubMed] [Google Scholar]
- 9.Zhang L, Tarleton R L. Eur J Immunol. 1996;26:102–109. doi: 10.1002/eji.1830260116. [DOI] [PubMed] [Google Scholar]
- 10.Zhang L, Tarleton R L. Exp Parasitol. 1996;84:203–213. doi: 10.1006/expr.1996.0106. [DOI] [PubMed] [Google Scholar]
- 11.Nuovo G J. PCR in Situ Hybridization Protocols and Applications. New York: Raven; 1994. [Google Scholar]
- 12.Degrave W, Fragoso S P, Britto C, van Heuverswyn H, Kidane G Z, Cardoso M A B, Mueller R U, Simpson L, Morel C M. Mol Biochem Parasitol. 1988;27:63–70. doi: 10.1016/0166-6851(88)90025-4. [DOI] [PubMed] [Google Scholar]
- 13.Postan M, Cheever A W, Dvorak J A, McDaniel J P. Trans R Soc Trop Med Hyg. 1985;80:50–55. doi: 10.1016/0035-9203(86)90193-8. [DOI] [PubMed] [Google Scholar]
- 14.Rossi M A. Am J Pathol. 1992;141:183–191. [PMC free article] [PubMed] [Google Scholar]
- 15.Tarleton R L. In: Immunology and Molecular Biology of Parasitic Infections. 3rd Ed. Warren K W, editor. Boston: Blackwell; 1993. pp. 64–71. [Google Scholar]
- 16.Sato M N, Yamashiro K E, Tanji M M, Kaneno R, Higuchi M L, Duarte A J. Infect Immun. 1992;60:1024–1030. doi: 10.1128/iai.60.3.1024-1030.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.d’Avila Reis D, Gazzinelli R T, Gazzinelli G, Colley D G. J Immunol. 1993;150:1611–1618. [PubMed] [Google Scholar]
- 18.Sun J, Tarleton R L. Am J Trop Med Hyg. 1993;48:161–169. doi: 10.4269/ajtmh.1993.48.161. [DOI] [PubMed] [Google Scholar]
- 19.Tarleton R L, Sun J, Zhang L, Postan M. Infect Immun. 1994;62:1820–1829. doi: 10.1128/iai.62.5.1820-1829.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nabors G S, Tarleton R L. J Immunol. 1991;146:3591–3598. [PubMed] [Google Scholar]
- 21.Torrico F, Heremans H, Rivera M T, Van Marck E, Billiau A, Carlier Y. J Immunol. 1991;146:3626–3632. [PubMed] [Google Scholar]
- 22.Tarleton R L. Clin Exp Immunol. 1988;73:186–190. [PMC free article] [PubMed] [Google Scholar]
- 23.Rivera M T, De Araujo S, Lucas R, Deman J, Truyens C, Defresne M P, De Baetselier P, Carlier Y. Infect Immun. 1995;63:591–595. doi: 10.1128/iai.63.2.591-595.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meckert P C, Hontebeyrie J M, Chambo J, Levin M, Laguens R P. Exp Parasitol. 1991;72:8–14. doi: 10.1016/0014-4894(91)90115-d. [DOI] [PubMed] [Google Scholar]
- 25.Postan M, Dvorak J A, McDaniel J P. Am J Trop Med Hyg. 1983;32:497–506. doi: 10.4269/ajtmh.1983.32.497. [DOI] [PubMed] [Google Scholar]
- 26.Postan M, Bailey J J, Dvorak J A, McDaniel J P, Pottala E W. Am J Trop Med Hyg. 1987;37:541–548. doi: 10.4269/ajtmh.1987.37.541. [DOI] [PubMed] [Google Scholar]
- 27.Minter-Goedbloed E, Minter D M, Marshall T Fd C. Trans R Soc Trop Med Hyg. 1978;72:217–225. doi: 10.1016/0035-9203(78)90196-7. [DOI] [PubMed] [Google Scholar]
- 28.Mirkin G A, Jones M, Sanz O P, Rey R, Sica R E, Gonzalez-Cappa S. Clin Immunol Immunopathol. 1994;73:69–79. doi: 10.1006/clin.1994.1171. [DOI] [PubMed] [Google Scholar]
- 29.Lima M T, Lenzi H L, Gattass C R. Parasitol Res. 1995;81:6–12. doi: 10.1007/BF00932410. [DOI] [PubMed] [Google Scholar]
- 30.Ben Younes-Chennoufi A, Hontebeyrie-Joskowicz M, Tricottet V, Eisen H, Reynes M, Said G. Trans R Soc Trop Med Hyg. 1988;82:77–83. doi: 10.1016/0035-9203(88)90269-6. [DOI] [PubMed] [Google Scholar]
- 31.Jones E M, Colley D G, Tostes S, Lopes E R, Vnencak J C, McCurley T L. Am J Trop Med Hyg. 1993;48:348–357. doi: 10.4269/ajtmh.1993.48.348. [DOI] [PubMed] [Google Scholar]
- 32.Higuchi Md L, de Brito T, Reis M M, Barbosa A, Bellotti G, Pereira-Barreto A C, Pileggi F. Cardiovasc Pathol. 1993;2:101–106. doi: 10.1016/1054-8807(93)90021-S. [DOI] [PubMed] [Google Scholar]
- 33.Bellotti G, Bocchi E A, de Moraes A V, Higuchi M D L, Barbero-Marchial M, Sosa E, Esteves-Filho A, Kahil R, Weiss R, Jatene A, Pileggi F. Am Heart J. 1996;131:301–307. doi: 10.1016/s0002-8703(96)90358-0. [DOI] [PubMed] [Google Scholar]
- 34.Andrade S G, Stocker G S, Pimentel A S, Grimaud J A. Mem Inst Oswaldo Cruz. 1991;86:187–200. doi: 10.1590/s0074-02761991000200008. [DOI] [PubMed] [Google Scholar]
- 35.Andrade S G, Rassi A, Magalhaes J B, Ferriolli F F, Luquetti A O. Trans R Soc Trop Med Hyg. 1992;86:624–626. doi: 10.1016/0035-9203(92)90156-7. [DOI] [PubMed] [Google Scholar]
- 36.Viotti R, Vigliano C, Armenti H, Segura E. Am Heart J. 1994;127:151–162. doi: 10.1016/0002-8703(94)90521-5. [DOI] [PubMed] [Google Scholar]
- 37.Silva J S, Rossi M A. J Exp Pathol. 1990;71:33–39. [PMC free article] [PubMed] [Google Scholar]
- 38.Jardim E, Takayanagui O M. Am J Trop Med Hyg. 1994;97:367–370. [PubMed] [Google Scholar]
- 39.Rottenberg M E, Riarte A, Sporrong L, Altcheh J, Petray P, Ruiz A M, Wigzell H, Orn A. Immunol Lett. 1995;45:53–60. doi: 10.1016/0165-2478(94)00221-c. [DOI] [PubMed] [Google Scholar]
- 40.Tarleton R L, Grusby M J, Postan M, Glimcher L H. Int Immunol. 1996;8:13–22. doi: 10.1093/intimm/8.1.13. [DOI] [PubMed] [Google Scholar]