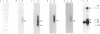Abstract
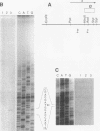
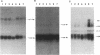
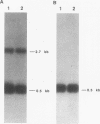
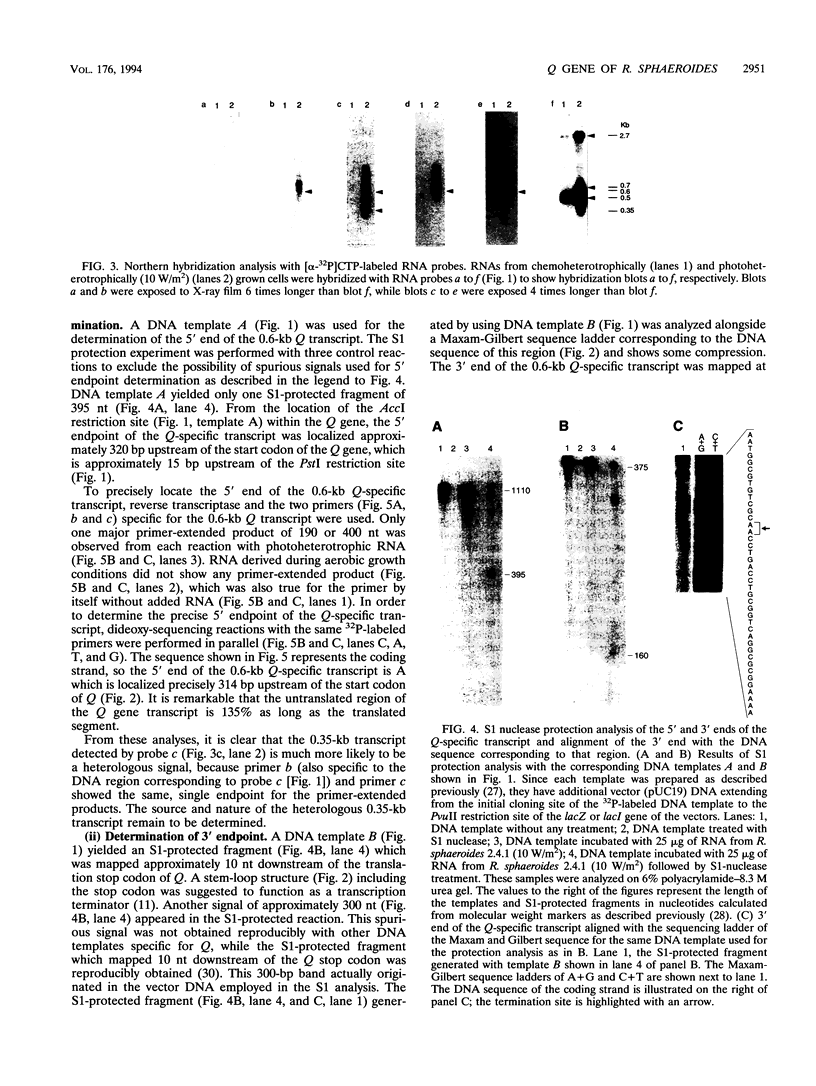
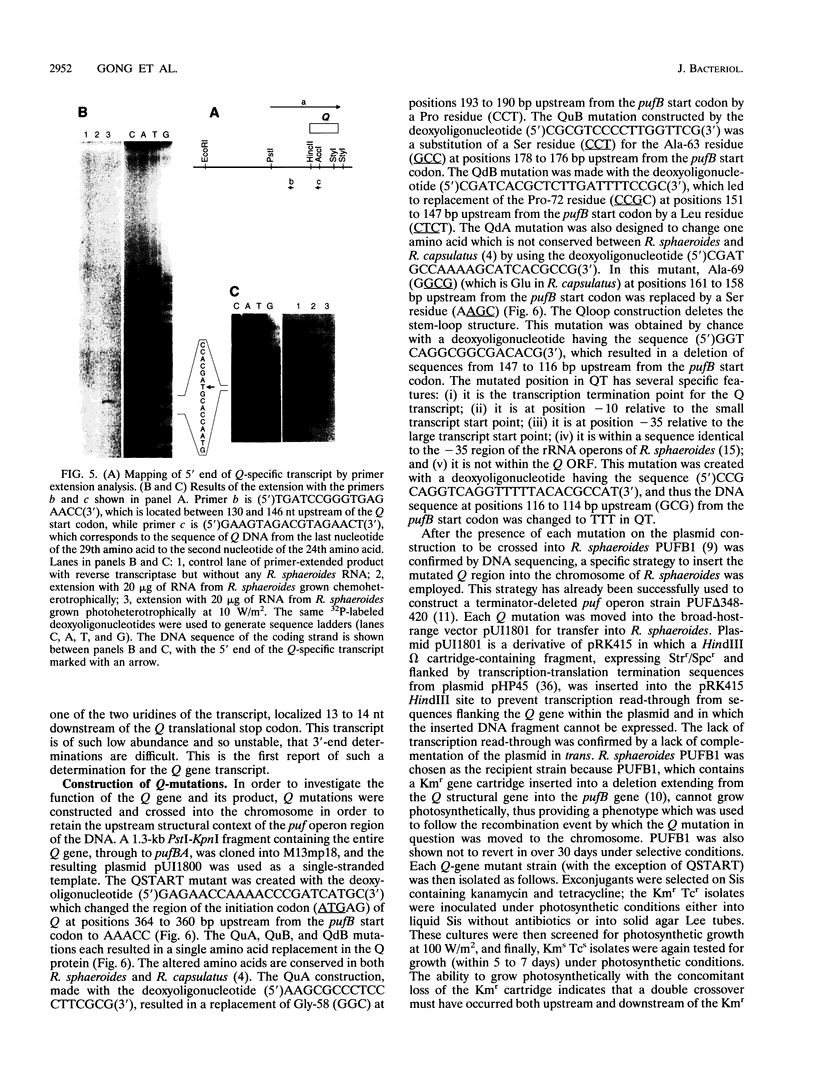
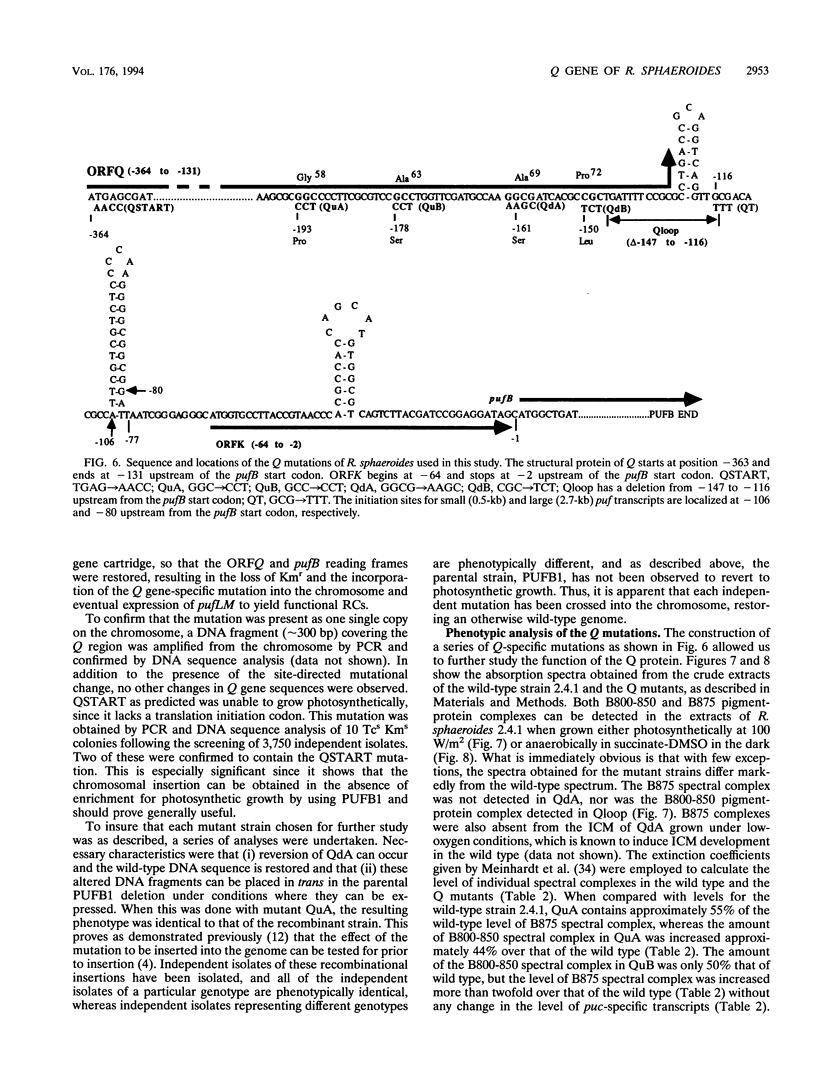
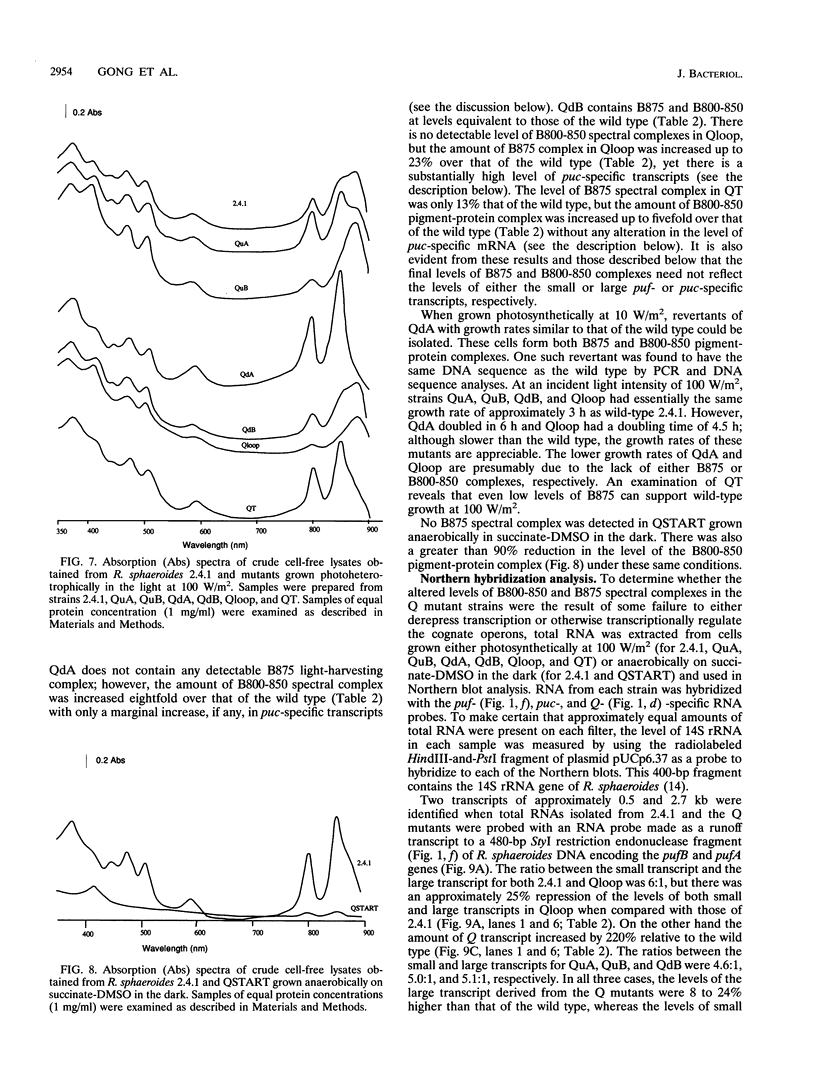
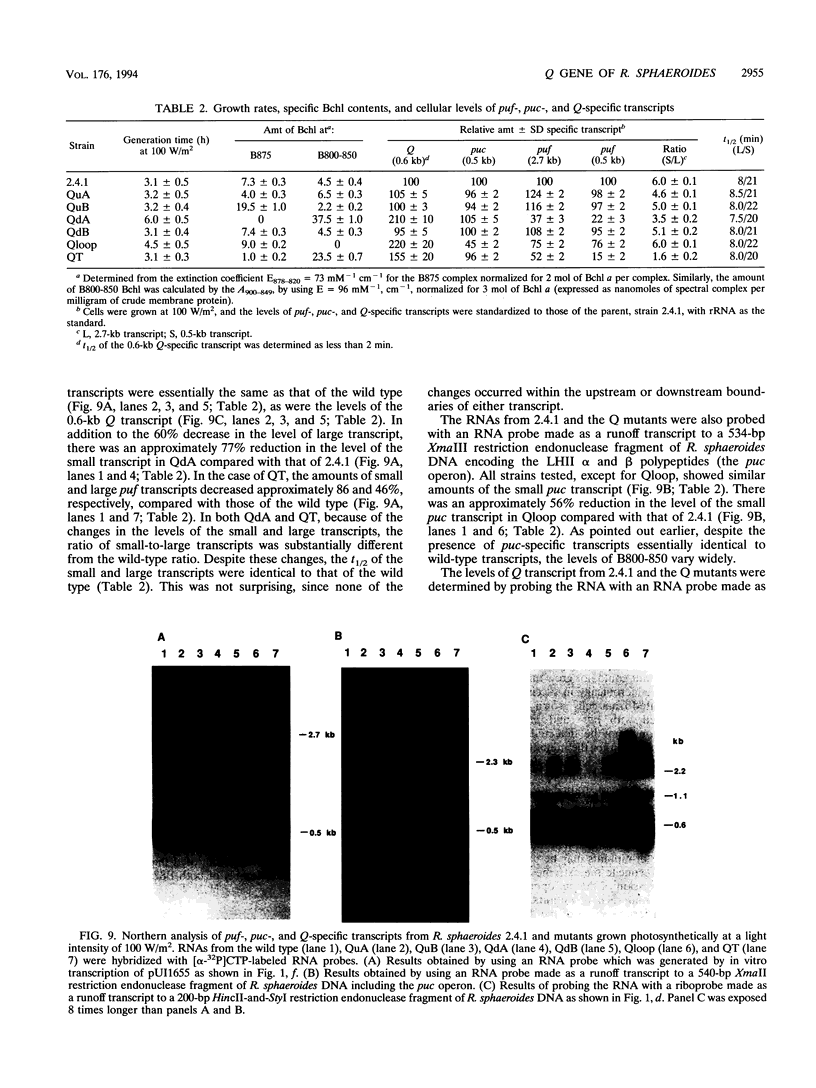
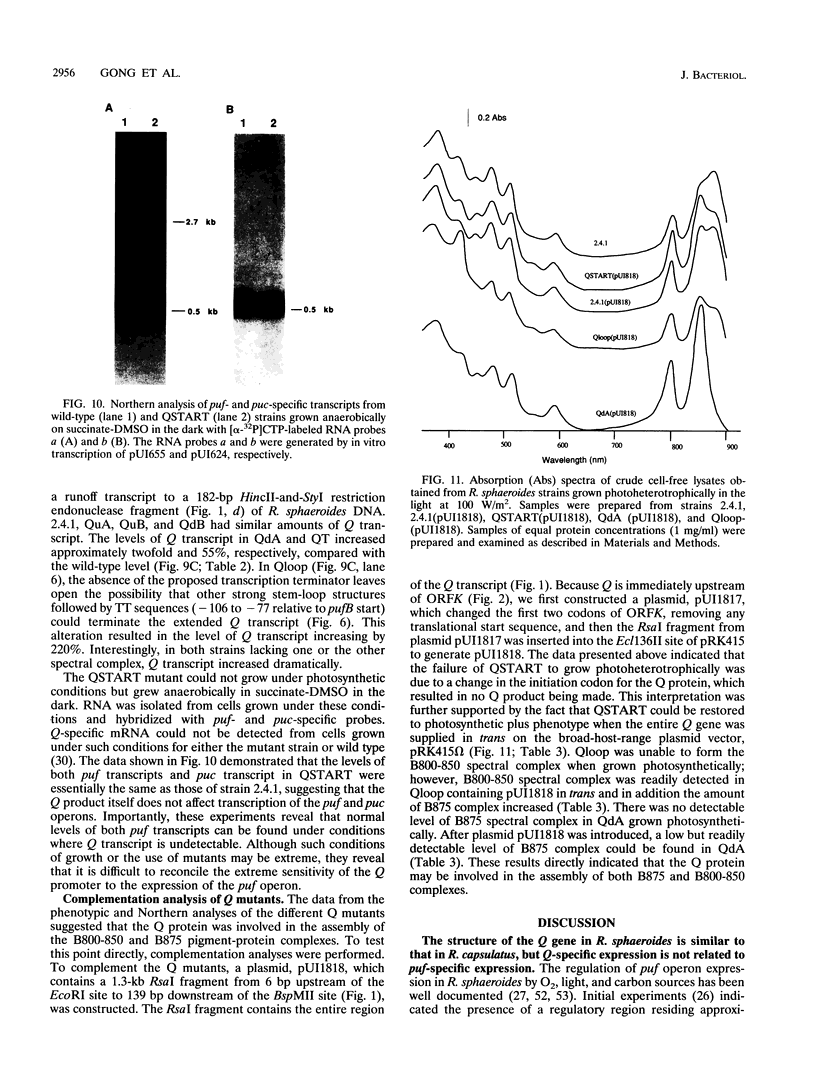
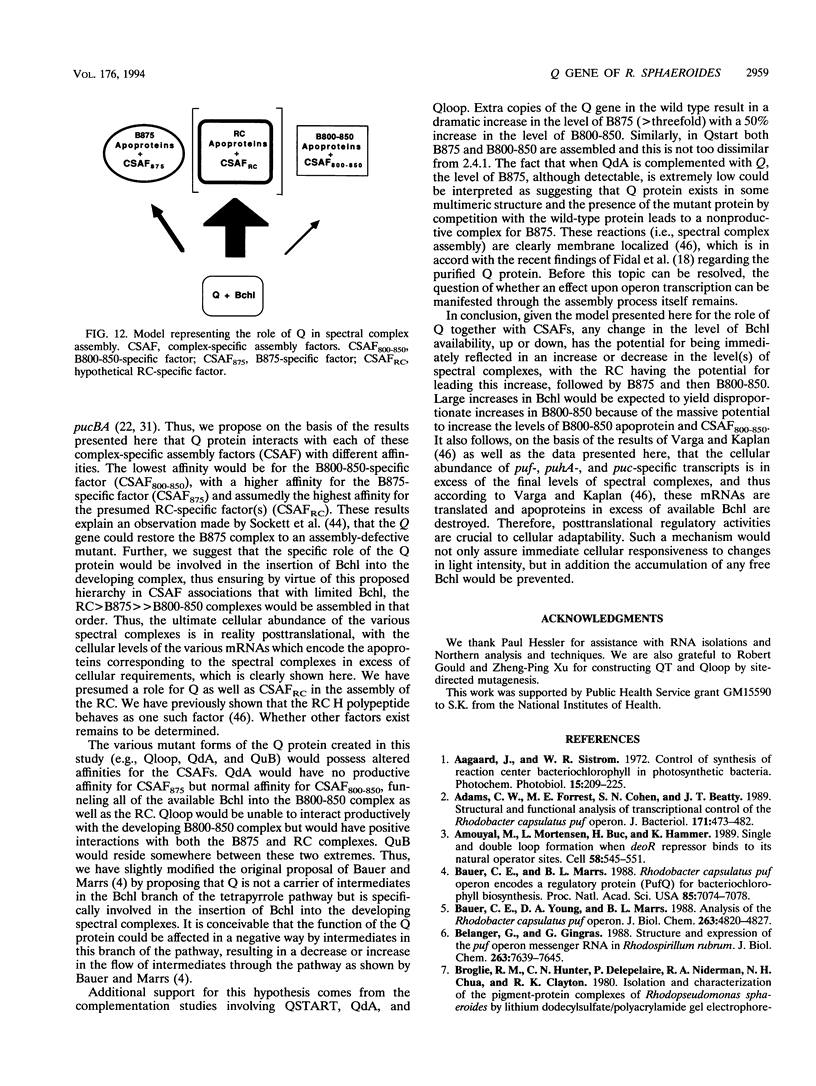
The Q gene of the facultative photoheterotroph Rhodobacter sphaeroides, localized immediately upstream of the oxygen- and light-regulated puf operon, encodes a 77-amino-acid polypeptide. The 5' and 3' ends of the 561-bp Q transcript were determined. To gain insight into the role of the Q gene product, a number of Q mutations were constructed by oligonucleotide-directed mutagenesis and subsequent substitution of the mutated form of the gene in single copy for the chromosomal copy via homologous recombination. The resulting mutants can grow photosynthetically, with the exception of QSTART, in which the initiation codon for the Q protein was altered. Spectral analysis of the intracytoplasmic membranes showed that one of the missense mutants (QdA) was deficient in the formation of detectable B875 light-harvesting complex (LHC), whereas deletion of the stem-loop structure (Qloop) failed to form B800-850 LHC when grown anaerobically either in the dark or under light intensity of 100 W/m2. Other missense mutants (QuA and QuB) contained either more B800-850 LHC or more B875 LHC, respectively, than the wild type. Although the levels of puf and puc transcripts isolated from QSTART grown anaerobically on succinate-dimethyl sulfoxide in the dark were comparable to wild-type levels, no B875 spectral complex was detected and there was a greater than 90% reduction in the level of the B800-850 pigment-protein complex. It has also been confirmed that the ultimate cellular levels of either the B875 or B800-850 spectral complexes can vary over wide limits without any change in the level(s) of complex specific transcripts. When the wild-type Q gene was reintroduced in trans into the Q mutations, QSTART was able to grow photosynthetically and both B800-850 and B875 spectral complexes were formed in either QdA or Qloop. Finally, we demonstrated that the level of each puf-specific mRNA behaves independently of one another as well as independently of the level(s) of Q gene-specific mRNA. These results are compatible with the existence of regulatory sequences affecting the puf mRNA level(s) being localized within the Q structural gene. These results suggest that Q-specific expression is uncoupled from puf-specific transcription and that the Q protein is not involved in the regulation of transcription of the puf operon but is directly involved in the assembly of both the B875 and B800-850 pigment-protein complexes.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aagaard J., Sistrom W. R. Control of synthesis of reaction center bacteriochlorophyll in photosynthetic bacteria. Photochem Photobiol. 1972 Feb;15(2):209–225. doi: 10.1111/j.1751-1097.1972.tb06240.x. [DOI] [PubMed] [Google Scholar]
- Adams C. W., Forrest M. E., Cohen S. N., Beatty J. T. Structural and functional analysis of transcriptional control of the Rhodobacter capsulatus puf operon. J Bacteriol. 1989 Jan;171(1):473–482. doi: 10.1128/jb.171.1.473-482.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amouyal M., Mortensen L., Buc H., Hammer K. Single and double loop formation when deoR repressor binds to its natural operator sites. Cell. 1989 Aug 11;58(3):545–551. doi: 10.1016/0092-8674(89)90435-2. [DOI] [PubMed] [Google Scholar]
- Bauer C. E., Marrs B. L. Rhodobacter capsulatus puf operon encodes a regulatory protein (PufQ) for bacteriochlorophyll biosynthesis. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7074–7078. doi: 10.1073/pnas.85.19.7074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer C. E., Young D. A., Marrs B. L. Analysis of the Rhodobacter capsulatus puf operon. Location of the oxygen-regulated promoter region and the identification of an additional puf-encoded gene. J Biol Chem. 1988 Apr 5;263(10):4820–4827. [PubMed] [Google Scholar]
- Burke D. H., Alberti M., Hearst J. E. The Rhodobacter capsulatus chlorin reductase-encoding locus, bchA, consists of three genes, bchX, bchY, and bchZ. J Bacteriol. 1993 Apr;175(8):2407–2413. doi: 10.1128/jb.175.8.2407-2413.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bélanger G., Gingras G. Structure and expression of the puf operon messenger RNA in rhodospirillum rubrum. J Biol Chem. 1988 Jun 5;263(16):7639–7645. [PubMed] [Google Scholar]
- Davis J., Donohue T. J., Kaplan S. Construction, characterization, and complementation of a Puf- mutant of Rhodobacter sphaeroides. J Bacteriol. 1988 Jan;170(1):320–329. doi: 10.1128/jb.170.1.320-329.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeHoff B. S., Lee J. K., Donohue T. J., Gumport R. I., Kaplan S. In vivo analysis of puf operon expression in Rhodobacter sphaeroides after deletion of a putative intercistronic transcription terminator. J Bacteriol. 1988 Oct;170(10):4681–4692. doi: 10.1128/jb.170.10.4681-4692.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dryden S. C., Kaplan S. Identification of cis-acting regulatory regions upstream of the rRNA operons of Rhodobacter sphaeroides. J Bacteriol. 1993 Oct;175(20):6392–6402. doi: 10.1128/jb.175.20.6392-6402.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dryden S. C., Kaplan S. Localization and structural analysis of the ribosomal RNA operons of Rhodobacter sphaeroides. Nucleic Acids Res. 1990 Dec 25;18(24):7267–7277. doi: 10.1093/nar/18.24.7267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farchaus J. W., Barz W. P., Grünberg H., Oesterhelt D. Studies on the expression of the pufX polypeptide and its requirement for photoheterotrophic growth in Rhodobacter sphaeroides. EMBO J. 1992 Aug;11(8):2779–2788. doi: 10.1002/j.1460-2075.1992.tb05345.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidai S., Kalmar G. B., Richards W. R., Borgford T. J. Recombinant expression of the pufQ gene of Rhodobacter capsulatus. J Bacteriol. 1993 Aug;175(15):4834–4842. doi: 10.1128/jb.175.15.4834-4842.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara S., Zielinski N. A., Chakrabarty A. M. Enhancer-like activity of A1gR1-binding site in alginate gene activation: positional, orientational, and sequence specificity. J Bacteriol. 1993 Sep;175(17):5452–5459. doi: 10.1128/jb.175.17.5452-5459.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter C. N., McGlynn P., Ashby M. K., Burgess J. G., Olsen J. D. DNA sequencing and complementation/deletion analysis of the bchA-puf operon region of Rhodobacter sphaeroides: in vivo mapping of the oxygen-regulated puf promoter. Mol Microbiol. 1991 Nov;5(11):2649–2661. doi: 10.1111/j.1365-2958.1991.tb01974.x. [DOI] [PubMed] [Google Scholar]
- Jones M. R., Fowler G. J., Gibson L. C., Grief G. G., Olsen J. D., Crielaard W., Hunter C. N. Mutants of Rhodobacter sphaeroides lacking one or more pigment-protein complexes and complementation with reaction-centre, LH1, and LH2 genes. Mol Microbiol. 1992 May;6(9):1173–1184. doi: 10.1111/j.1365-2958.1992.tb01556.x. [DOI] [PubMed] [Google Scholar]
- Keen N. T., Tamaki S., Kobayashi D., Trollinger D. Improved broad-host-range plasmids for DNA cloning in gram-negative bacteria. Gene. 1988 Oct 15;70(1):191–197. doi: 10.1016/0378-1119(88)90117-5. [DOI] [PubMed] [Google Scholar]
- Kiley P. J., Donohue T. J., Havelka W. A., Kaplan S. DNA sequence and in vitro expression of the B875 light-harvesting polypeptides of Rhodobacter sphaeroides. J Bacteriol. 1987 Feb;169(2):742–750. doi: 10.1128/jb.169.2.742-750.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiley P. J., Kaplan S. Molecular genetics of photosynthetic membrane biosynthesis in Rhodobacter sphaeroides. Microbiol Rev. 1988 Mar;52(1):50–69. doi: 10.1128/mr.52.1.50-69.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. K., DeHoff B. S., Donohue T. J., Gumport R. I., Kaplan S. Transcriptional analysis of puf operon expression in Rhodobacter sphaeroides 2.4.1 and an intercistronic transcription terminator mutant. J Biol Chem. 1989 Nov 15;264(32):19354–19365. [PubMed] [Google Scholar]
- Lee J. K., Kaplan S. cis-acting regulatory elements involved in oxygen and light control of puc operon transcription in Rhodobacter sphaeroides. J Bacteriol. 1992 Feb;174(4):1146–1157. doi: 10.1128/jb.174.4.1146-1157.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. K., Kiley P. J., Kaplan S. Posttranscriptional control of puc operon expression of B800-850 light-harvesting complex formation in Rhodobacter sphaeroides. J Bacteriol. 1989 Jun;171(6):3391–3405. doi: 10.1128/jb.171.6.3391-3405.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McGlynn P., Hunter C. N. Genetic analysis of the bchC and bchA genes of Rhodobacter sphaeroides. Mol Gen Genet. 1993 Jan;236(2-3):227–234. doi: 10.1007/BF00277117. [DOI] [PubMed] [Google Scholar]
- Meinhardt S. W., Kiley P. J., Kaplan S., Crofts A. R., Harayama S. Characterization of light-harvesting mutants of Rhodopseudomonas sphaeroides. I. Measurement of the efficiency of energy transfer from light-harvesting complexes to the reaction center. Arch Biochem Biophys. 1985 Jan;236(1):130–139. doi: 10.1016/0003-9861(85)90612-5. [DOI] [PubMed] [Google Scholar]
- Neidle E. L., Kaplan S. Expression of the Rhodobacter sphaeroides hemA and hemT genes, encoding two 5-aminolevulinic acid synthase isozymes. J Bacteriol. 1993 Apr;175(8):2292–2303. doi: 10.1128/jb.175.8.2292-2303.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prentki P., Krisch H. M. In vitro insertional mutagenesis with a selectable DNA fragment. Gene. 1984 Sep;29(3):303–313. doi: 10.1016/0378-1119(84)90059-3. [DOI] [PubMed] [Google Scholar]
- Reitzer L. J., Movsas B., Magasanik B. Activation of glnA transcription by nitrogen regulator I (NRI)-phosphate in Escherichia coli: evidence for a long-range physical interaction between NRI-phosphate and RNA polymerase. J Bacteriol. 1989 Oct;171(10):5512–5522. doi: 10.1128/jb.171.10.5512-5522.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SISTROM W. R. The kinetics of the synthesis of photopigments in Rhodopseudomonas spheroides. J Gen Microbiol. 1962 Sep;28:607–616. doi: 10.1099/00221287-28-4-607. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada H., Ohta H., Masuda T., Shioi Y., Takamiya K. A putative transcription factor binding to the upstream region of the puf operon in Rhodobacter sphaeroides. FEBS Lett. 1993 Aug 9;328(1-2):41–44. doi: 10.1016/0014-5793(93)80961-s. [DOI] [PubMed] [Google Scholar]
- Smith R. W., Masters M., Donachie W. D. Cell division and transcription of ftsZ. J Bacteriol. 1993 May;175(9):2788–2791. doi: 10.1128/jb.175.9.2788-2791.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sockett R. E., Donohue T. J., Varga A. R., Kaplan S. Control of photosynthetic membrane assembly in Rhodobacter sphaeroides mediated by puhA and flanking sequences. J Bacteriol. 1989 Jan;171(1):436–446. doi: 10.1128/jb.171.1.436-446.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga A. R., Kaplan S. Synthesis and stability of reaction center polypeptides and implications for reaction center assembly in Rhodobacter sphaeroides. J Biol Chem. 1993 Sep 15;268(26):19842–19850. [PubMed] [Google Scholar]
- Wang X. D., de Boer P. A., Rothfield L. I. A factor that positively regulates cell division by activating transcription of the major cluster of essential cell division genes of Escherichia coli. EMBO J. 1991 Nov;10(11):3363–3372. doi: 10.1002/j.1460-2075.1991.tb04900.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiessner C., Dunger I., Michel H. Structure and transcription of the genes encoding the B1015 light-harvesting complex beta and alpha subunits and the photosynthetic reaction center L, M, and cytochrome c subunits from Rhodopseudomonas viridis. J Bacteriol. 1990 Jun;172(6):2877–2887. doi: 10.1128/jb.172.6.2877-2887.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. C., Steiner L. A., Feher G., Simon M. I. Primary structure of the L subunit of the reaction center from Rhodopseudomonas sphaeroides. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7303–7307. doi: 10.1073/pnas.81.23.7303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. C., Steiner L. A., Ogden R. C., Simon M. I., Feher G. Primary structure of the M subunit of the reaction center from Rhodopseudomonas sphaeroides. Proc Natl Acad Sci U S A. 1983 Nov;80(21):6505–6509. doi: 10.1073/pnas.80.21.6505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y. S., Kaplan S. Effects of light, oxygen, and substrates on steady-state levels of mRNA coding for ribulose-1,5-bisphosphate carboxylase and light-harvesting and reaction center polypeptides in Rhodopseudomonas sphaeroides. J Bacteriol. 1985 Jun;162(3):925–932. doi: 10.1128/jb.162.3.925-932.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y. S., Kiley P. J., Donohue T. J., Kaplan S. Origin of the mRNA stoichiometry of the puf operon in Rhodobacter sphaeroides. J Biol Chem. 1986 Aug 5;261(22):10366–10374. [PubMed] [Google Scholar]
- de Smit M. H., van Duin J. Translational initiation at the coat-protein gene of phage MS2: native upstream RNA relieves inhibition by local secondary structure. Mol Microbiol. 1993 Sep;9(5):1079–1088. doi: 10.1111/j.1365-2958.1993.tb01237.x. [DOI] [PubMed] [Google Scholar]
- van Niel C. B. THE CULTURE, GENERAL PHYSIOLOGY, MORPHOLOGY, AND CLASSIFICATION OF THE NON-SULFUR PURPLE AND BROWN BACTERIA. Bacteriol Rev. 1944 Mar;8(1):1–118. doi: 10.1128/br.8.1.1-118.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]