Abstract
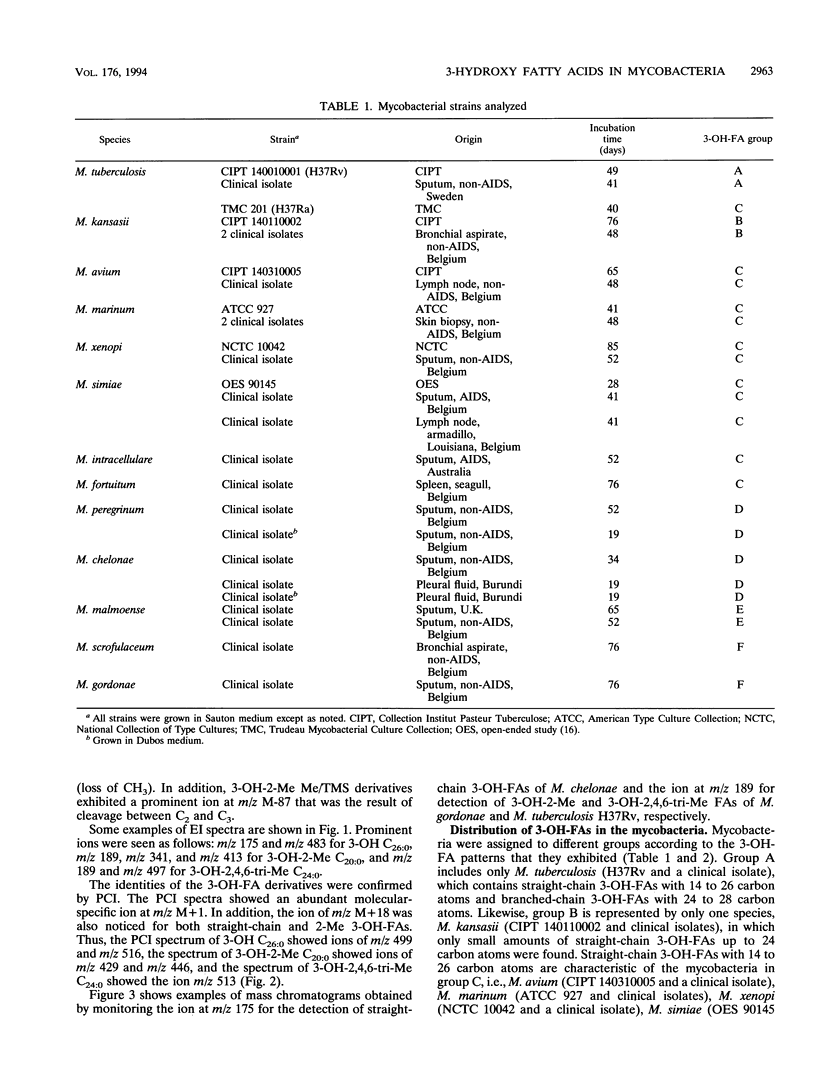
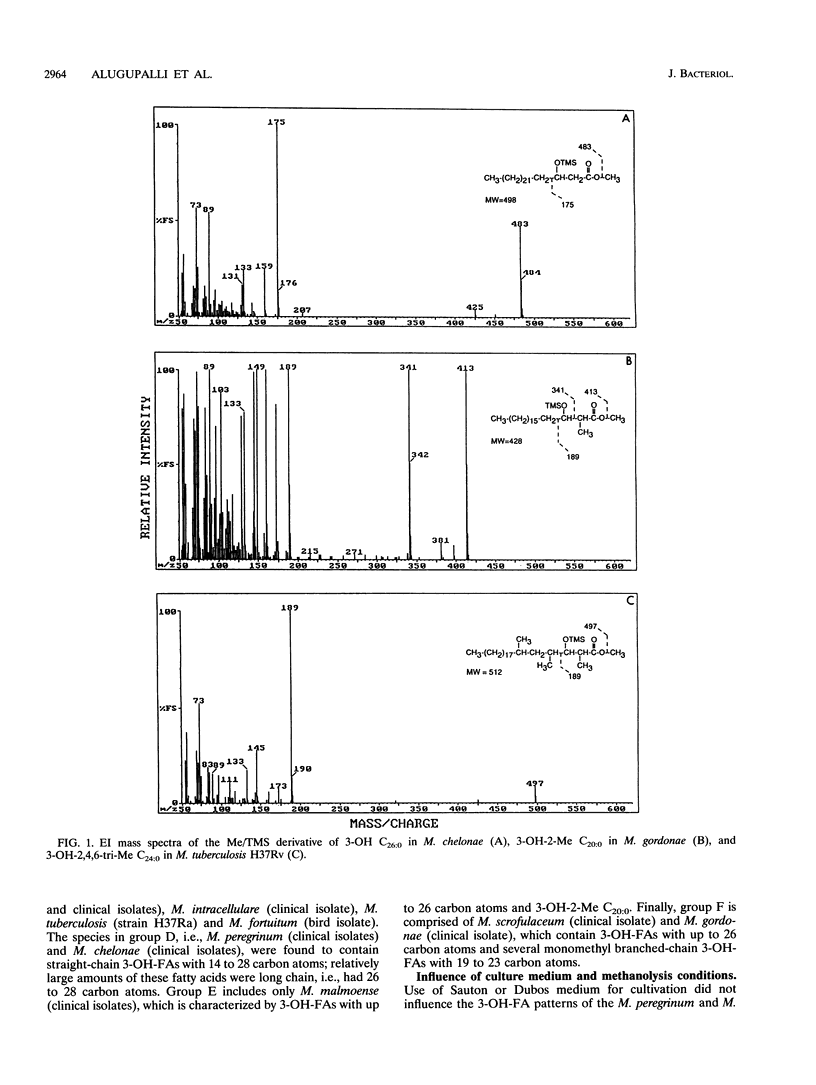
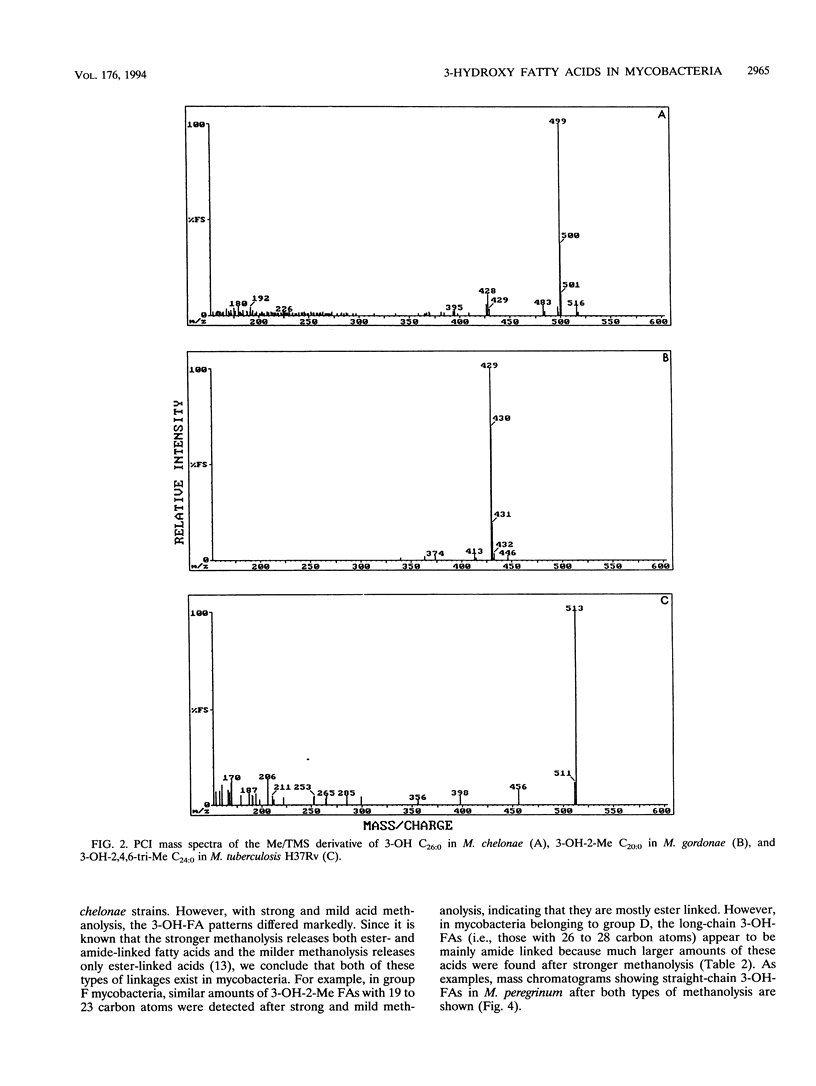
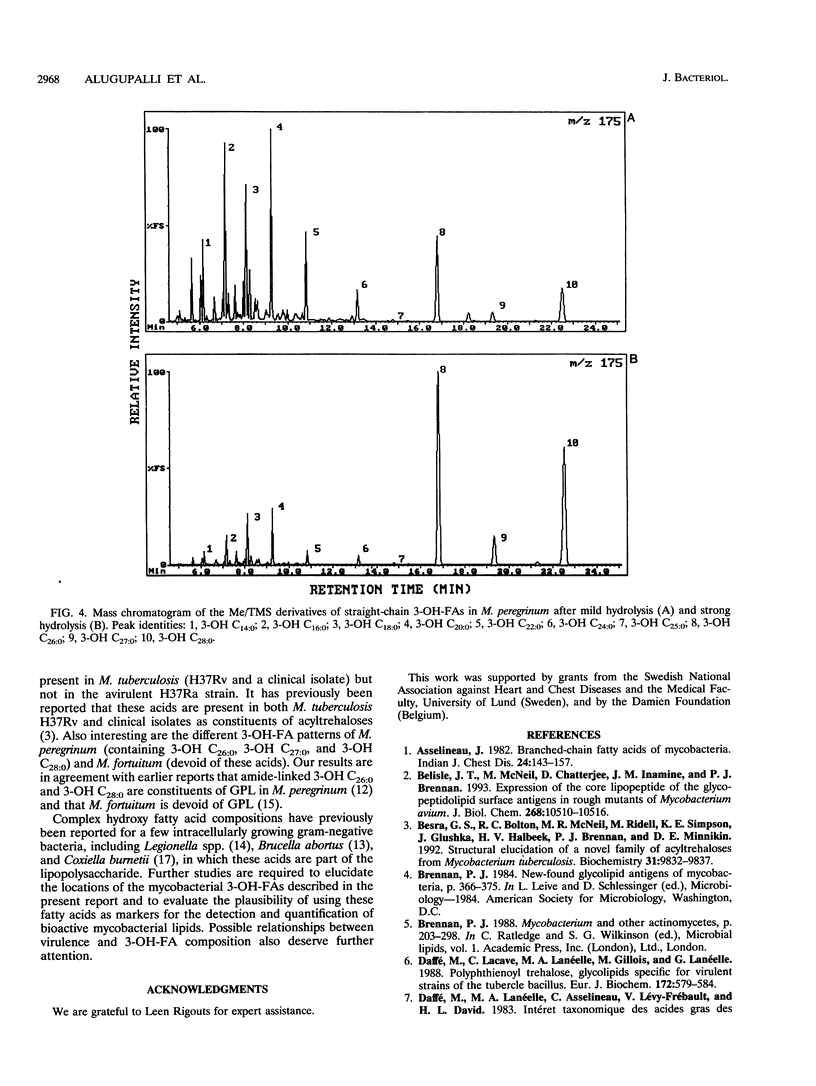
Twenty-seven strains belonging to 12 Mycobacterium species were studied for 3-hydroxy fatty acid composition. Mycobacterial cells were subjected to both mild and strong acid methanolysis, after which the liberated hydroxy fatty acids were purified and analyzed by gas chromatography-mass spectrometry as methyl ester trimethylsilyl ether derivatives. Altogether, 21 3-hydroxy fatty acids containing 14 to 28 carbon atoms were detected; 10 were straight chain, 6 were 2-methyl branched chain, and 5 were 2,4,6-trimethyl branched chain. The mycobacterial strains were classified in groups according to 3-hydroxy fatty acid patterns.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asselineau J. Branched-chain fatty acids of mycobacteria. Indian J Chest Dis Allied Sci. 1982 Apr-Sep;24(2-3):143–157. [PubMed] [Google Scholar]
- Belisle J. T., McNeil M. R., Chatterjee D., Inamine J. M., Brennan P. J. Expression of the core lipopeptide of the glycopeptidolipid surface antigens in rough mutants of Mycobacterium avium. J Biol Chem. 1993 May 15;268(14):10510–10516. [PubMed] [Google Scholar]
- Besra G. S., Bolton R. C., McNeil M. R., Ridell M., Simpson K. E., Glushka J., van Halbeek H., Brennan P. J., Minnikin D. E. Structural elucidation of a novel family of acyltrehaloses from Mycobacterium tuberculosis. Biochemistry. 1992 Oct 13;31(40):9832–9837. doi: 10.1021/bi00155a040. [DOI] [PubMed] [Google Scholar]
- Daffe M., Laneelle M. A., Puzo G. Structural elucidation by field desorption and electron-impact mass spectrometry of the C-mycosides isolated from Mycobacterium smegmatis. Biochim Biophys Acta. 1983 May 16;751(3):439–443. doi: 10.1016/0005-2760(83)90304-1. [DOI] [PubMed] [Google Scholar]
- Daffe M., McNeil M., Brennan P. J. Novel type-specific lipooligosaccharides from Mycobacterium tuberculosis. Biochemistry. 1991 Jan 15;30(2):378–388. doi: 10.1021/bi00216a011. [DOI] [PubMed] [Google Scholar]
- Daffé M., Lacave C., Lanéelle M. A., Gillois M., Lanéelle G. Polyphthienoyl trehalose, glycolipids specific for virulent strains of the tubercle bacillus. Eur J Biochem. 1988 Mar 15;172(3):579–584. doi: 10.1111/j.1432-1033.1988.tb13928.x. [DOI] [PubMed] [Google Scholar]
- Hunter S. W., Barr V. L., McNeil M., Jardine I., Brennan P. J. Trehalose-containing lipooligosaccharide antigens of Mycobacterium sp.: presence of a mono-O-methyltri-O-acyltrehalose "core". Biochemistry. 1988 Mar 8;27(5):1549–1556. doi: 10.1021/bi00405a023. [DOI] [PubMed] [Google Scholar]
- Lanéelle M. A., Promé D., Lanéelle G., Promé J. C. Ornithine lipid of Mycobacterium tuberculosis: its distribution in some slow- and fast-growing mycobacteria. J Gen Microbiol. 1990 Apr;136(4):773–778. doi: 10.1099/00221287-136-4-773. [DOI] [PubMed] [Google Scholar]
- López Marin L. M., Lanéelle M. A., Promé D., Daffé M., Lanéelle G., Promé J. C. Glycopeptidolipids from Mycobacterium fortuitum: a variant in the structure of C-mycoside. Biochemistry. 1991 Oct 29;30(43):10536–10542. doi: 10.1021/bi00107a024. [DOI] [PubMed] [Google Scholar]
- Moreno E., Stackebrandt E., Dorsch M., Wolters J., Busch M., Mayer H. Brucella abortus 16S rRNA and lipid A reveal a phylogenetic relationship with members of the alpha-2 subdivision of the class Proteobacteria. J Bacteriol. 1990 Jul;172(7):3569–3576. doi: 10.1128/jb.172.7.3569-3576.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonesson A., Jantzen E., Bryn K., Larsson L., Eng J. Chemical composition of a lipopolysaccharide from Legionella pneumophila. Arch Microbiol. 1989;153(1):72–78. doi: 10.1007/BF00277544. [DOI] [PubMed] [Google Scholar]
- Wayne L. G., Good R. C., Krichevsky M. I., Blacklock Z., David H. L., Dawson D., Gross W., Hawkins J., Levy-Frebault V. V., McManus C. Fourth report of the cooperative, open-ended study of slowly growing mycobacteria by the International Working Group on Mycobacterial Taxonomy. Int J Syst Bacteriol. 1991 Oct;41(4):463–472. doi: 10.1099/00207713-41-4-463. [DOI] [PubMed] [Google Scholar]
- Wollenweber H. W., Schramek S., Moll H., Rietschel E. T. Nature and linkage type of fatty acids present in lipopolysaccharides of phase I and II Coxiella burnetii. Arch Microbiol. 1985 Jun;142(1):6–11. doi: 10.1007/BF00409228. [DOI] [PubMed] [Google Scholar]



