Abstract
Mast cells derived from Bruton’s tyrosine kinase (Btk)-defective xid or btk null mice showed greater expansion in culture containing interleukin-3 (IL-3) than those from wild-type (wt) mice. Although the proliferative response to IL-3 was not significantly different between the wt and xid mast cells, xid and btk null mast cells died by apoptosis more slowly than their wt counterparts upon IL-3 deprivation. Consistent with these findings, the apoptosis-linked c-Jun N-terminal kinase/stress-activated protein kinase (JNK) activity was compromised in these btk-mutated cells upon FcɛRI crosslinking or upon stimulation with IL-3 or with stem cell factor. p38 activity was less severely, but significantly, affected by btk mutation, whereas extracellular signal-regulated kinases were not affected by the same mutation. Btk-mediated regulation of apoptosis and JNK activity was confirmed by reconstitution of btk null mutant mast cells with the wt btk cDNA. Furthermore, growth factor withdrawal induced the activation and sustained activity of JNK in wt mast cells, while JNK activity was consistently lower in btk-mutated mast cells. These results support the notion that Btk regulates apoptosis through the JNK activation.
Bruton’s tyrosine kinase (Btk) is a cytoplasmic protein-tyrosine kinase of the recently recognized Tec family (1–3). These protein-tyrosine kinases consist, from the N to C termini, of pleckstrin homology, Tec homology, Src homology 3 (SH3), SH2, and SH1 (=kinase) domains. Various defects in the btk gene cause X-linked agammaglobulinemia in humans (2, 3) and the Arg to Cys mutation at position 28 results in X-linked immunodeficiency (xid) in mice (4, 5). These diseases and the phenotype of btk gene knockout mice (6, 7) indicate an essential role of Btk in the development and functions of B lymphocytes. Our previous study showed that Btk is tyrosine-phosphorylated and enzymatically activated upon crosslinking of FcɛRI, suggesting a role of Btk in mast cell activation (8). Recent studies have made significant progresses in our understanding of how Btk activity is regulated. Thus, Btk was shown to be activated by phosphorylation of Tyr-551 by a Src family protein-tyrosine kinase, Lyn (9, 10). Activated Btk in turn autophosphorylates Tyr-223 in the SH3 domain (11). On the other hand, Btk is negatively regulated by protein kinase C in mast cells (12). Btk is also implicated in the regulation of intracellular Ca2+ concentrations through direct or indirect phosphorylation of phospholipase Cγ2 in chicken B lymphoma cells (13). However, the mechanisms by which Btk promotes normal functions in B cells and other cell types are largely unknown. In the present study, we provide evidence that Btk regulates the activity of c-Jun N terminal kinase or stress-activated protein kinase (JNK) and the apoptotic process upon growth factor deprivation of mast cells.
MATERIALS AND METHODS
Cells and Stimulation.
Bone marrow cells from femurs of CBA/J, CBA/CaHN-xid/J (xid), B6/129 F2, and btk null (6) mice were cultured in IL-3-containing RPMI 1640 medium/10% fetal calf serum with weekly changes of medium for 4–5 weeks before use (14). Cell density was adjusted to 3 × 105 cells per ml at the weekly change of medium. Numbers of live cells excluding trypan blue were counted by a hemocytometer. The phenotype of xid and btk null mast cells [xid-bone marrow-derived mouse mast cells (BMMC) and btk null-BMMC] was indistinguishable from wt mast cells in their morphology, as revealed by staining cells with May–Giemsa or with Alcian Blue (data not shown). Numbers of IgE binding sites on these mast cells at day 28 were also similar: 4.6 ± 1.2 × 104/cell (CBA/J) vs. 3.7 ± 2.0 × 104/cell (CBA/CaHN-xid/J). For FcɛRI crosslinking, BMMC primed with anti-dinitrophenyl IgE monoclonal antibody were incubated with dinitrophenyl conjugates of human serum albumin for the indicated intervals. For growth factor stimulation, BMMC were incubated in IL-3-free medium overnight before stimulation with recombinant mouse IL-3 or stem cell factor at 100 ng/ml (both from Kirin Brewery).
Transfection.
pMX-puro vector, which is a puromycin-resistant derivative of pMX-neo (15), was used to construct btk expression vectors. K430R and xid (R28C) mutant btk cdNAs were made by PCR-assisted in vitro mutagenesis and verified by a limited sequence determination. Transfection of Bosc-23 packaging cells (16) with these vectors was done with a Lipofectamine reagent (Life Technologies), and recovered retroviruses (3–10 × 105 colony-forming units/ml) were used to infect btk null-BMMC. Selection of transfected cells in the presence of 1.5 μg/ml puromycin for 2 to 3 weeks yielded sufficient numbers of cells for experiments.
Mitogen-Activated Protein Kinase (MAPK) Assays.
Extracellular signal-regulated kinases (ERKs). Cells were lysed, and fractions of lysates were directly analyzed by SDS/PAGE followed by immunoblotting with anti-phospho-MAPK (New England Biolabs). The same blot was reprobed with anti-p44/42 MAPK antibody (New England Biolabs) to confirm equal loadings. The rest of lysates were immunoprecipitated with anti-ERK1 (Zymed), and in vitro immune complex kinase assays were done using myelin basic protein (MBP) as a substrate in the presence of [γ-32P]ATP. Reaction products were analyzed by SDS/PAGE and autoradiography.
p38.
p38 immunoprecipitates were analyzed by immunoblotting with anti-phosphotyrosine antibody 4G10 (Upstate Biotechnology). The same blot was reprobed with anti-p38 (Santa Cruz Biotechnology) to control loadings. In in vitro kinase assays, immune complexes precipitated with anti-p38 (a gift from J. Han) were also incubated with MBP as substrate.
JNKs.
Kinase activity was measured on immune complexes precipitated with anti-JNK1 or anti-JNK2 using glutathione S-transferase (GST)-ATF-2 or GST-c-Jun(1–79) as substrate. Both antibodies were from Santa Cruz Biotechnology.
Analysis of Apoptosis and DNA Synthesis.
Mast cells depleted of growth factor for the indicated intervals were stained with propidium iodide and analyzed by FACScan (Becton Dickinson). Genomic DNAs were isolated and analyzed by agarose gel elctrophoresis. Mitogenic response of mast cells to IL-3 was measured by incubating cells (5 × 105/ml, 0.2 ml) in mouse recombinant IL-3 for 18 h in the presence of [3H]thymidine during the last 6 h. Thymidine incorporation into the acid-insoluble fraction was counted.
RESULTS
Defective Apoptosis of the btk-Mutated Mast Cells.
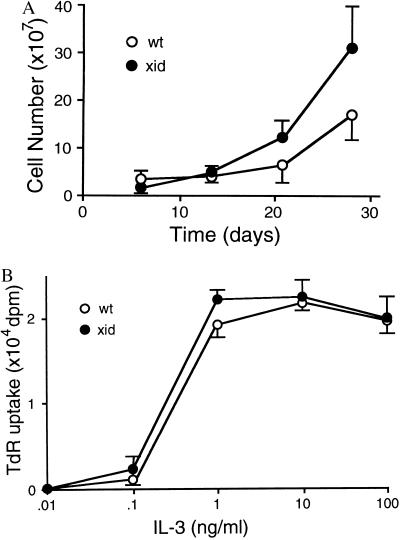
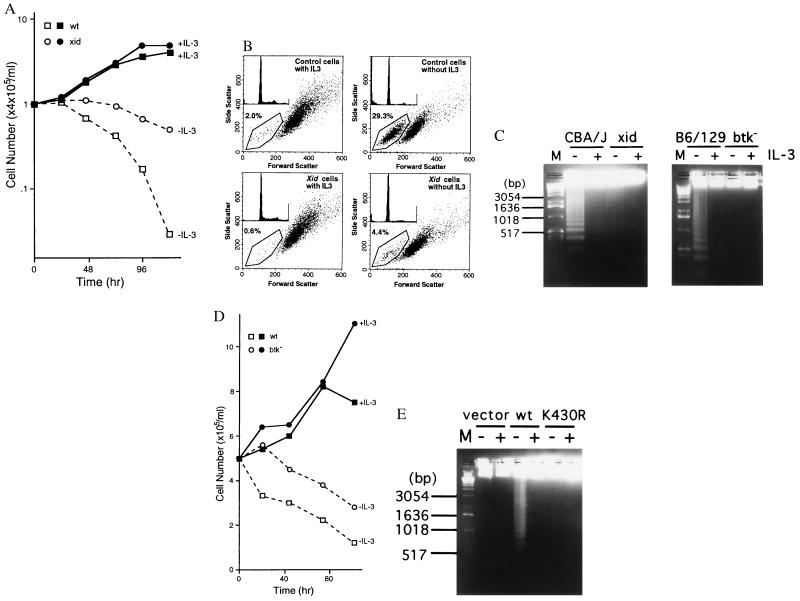
Culturing murine bone marrow cells in IL-3-containing medium for 4–5 weeks give rise to an almost pure (>95%) population of immature mast cells (BMMC). Starting from similar numbers of bone marrow cells, i.e., 2.0 ± 0.2 × 107 (xid) and 1.8 ± 0.1 × 107 (wt), numbers of BMMC obtained from xid mice were significantly greater than those from wt mice after 28 days of culture: 3.0 ± 0.8 × 108 (xid) vs. 1.6 ± 0.4 × 108 per femur (wt) (Fig. 1A), although the proliferative response of xid-BMMC to recombinant mouse IL-3 was not significantly different from that of wt-BMMC (Fig. 1B). Since medium was changed once a week during the culture of bone marrow cells, we considered the possibility of insufficient supply of IL-3. As shown previously (17), BMMCs die by apoptosis upon growth factor withdrawal. Cell survival was compared between wt- and btk-mutated mast cells under such conditions. Numbers of live xid-BMMC declined more slowly than those of wt-BMMC (Fig. 2A). Cytofluorometric analysis of propidium iodide-stained cells (Fig. 2B) and agarose gel electrophoresis of genomic DNAs (Fig. 2C) revealed that both wt- and xid-BMMCs die by apoptosis and that the apoptotic death of xid-BMMC is delayed by approximately 48 h compared with wt-BMMC. Similarly, btk null-BMMC died by apoptosis in the absence of IL-3 more slowly than wt-BMMC (Fig. 2 C and D). Furthermore, btk null-BMMC reconstituted with the wt btk cDNA underwent apoptosis more rapidly than btk null-BMMCs reconstituted with the vector or K430R btk cDNA, as revealed by earlier DNA fragmentation (Fig. 2E). Therefore, these results demonstrate that Btk regulates apoptosis induced by growth factor deprivation in mast cells.
Figure 1.
Growth of mast cells and their proliferative response to IL-3. (A) Growth curve of bone marrow-derived mast cells in IL-3-containing medium. Bone marrow cells obtained from CBA/J (wt) or CBA/CaHN-xid/J (xid) were cultured as described (14). Cell density was adjusted to 3 × 105 per ml weekly. Live cell numbers were counted weekly. Shown is a representative culture from more than 10 experiments. (B) BMMCs (5 × 105/ml, 0.2 ml/well) derived by 4 weeks of culture of bone marrow cells from CBA/J (wt) and CBA/CaHN-xid/J (xid) were cultured in the indicated concentrations of mouse recombinant IL-3 for 18 h in the presence of [3H]thymidine during the last 6 h. Thymidine incorporation into the acid-insoluble fraction was counted. Two similar experiments were performed with essentially the same results.
Figure 2.
xid and btk null mast cells die by apoptosis upon growth factor deprivation more slowly than the wt cells. (A) Survival curve of wt (CBA/J)- and xid-BMMCs in the presence or absence of IL-3 in the culture medium. Numbers of live cells excluding trypan blue were counted. Average values from duplicate samples are shown. Deviations between duplicate samples were less than 15% in all samples. Representative of six similar experiments. (B) Cytofluorometric analysis of propidium iodide-stained BMMCs. Cells were stained 30 h after IL-3 was removed from the medium. Percents of apoptotic cells are indicated. Insets show profiles of propidium iodide-stained cells. (C) DNA fragmentation was analyzed by agarose gel electrophoresis of genomic DNAs from BMMCs (4 × 105 cells) obtained before or 48 h (CBA/J and xid) or 10 h (B6/129 F2 and btk null) after growth factor deprivation. A 1-kb ladder (Life Technologies) was used as a molecular size marker (M). (D) Survival curve of wt (B6/129 F2)- and btk null-BMMCs in the presence or absence of IL-3. Experiments were done in duplicate with live cell numbers of less than 15% deviations among duplicates samples. Another experiment with a similar result was done. (E) DNA fragmentation was analyzed by agarose gel electrophoresis of genomic DNAs from btk null-BMMCs reconstituted with vector, wt, or K430Rbtk cDNAs prepared before (+) or 6 h after (−) growth factor deprivation.
Increased JNK Activity upon Growth Factor Withdrawal and Its Defect in btk null Mast Cells.
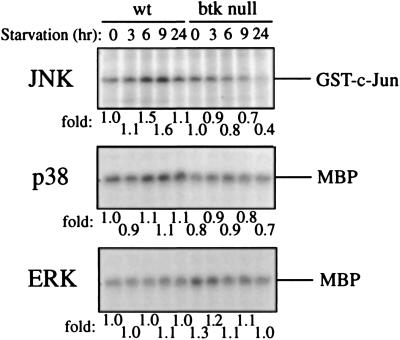
In a neuronal model system (18), apoptosis induced by growth factor deprivation was linked to the sustained activation of JNK and p38, while survival was controlled by the opposing effect of ERKs. Apoptosis induced by various stresses that activate the sphingomyelin pathway was shown to be dependent on the JNK pathway (19). Therefore, we measured JNK activity in wt- and btk null-BMMCs upon growth factor withdrawal (Fig. 3). JNK activity in wt-BMMC increased upon growth factor withdrawal. The peak activity of JNK1 was observed 6–9 h after the removal of growth factor, and JNK1 activity in wt-BMMC was higher than that in btk null-BMMC at any tested time point throughout the time course (0–24 hr). The activity of another MAPK, p38, in wt mast cells was also induced by growth factor deprivation, and it was also higher than that in btk null mast cells, whereas ERK1 activity was not induced by starvation, and it was slightly higher in btk null cells than that in wt cells (Fig. 3). These results are consistent with the notion that Btk regulates apoptosis by regulating JNK and p38.
Figure 3.
Activities of MAPK upon growth factor withdrawal. IL-3 was removed from cultures (2 × 106 cells) of wt- and btk null-BMMCs for the indicated intervals. Cells were lysed and immunoprecipitated with anti-JNK1, anti-p38, or anti-ERK1. Immune complex kinase assays were done, and reaction products were analyzed by SDS/PAGE and autoradiography. -Fold activities measured by densitometry of the substrate bands are shown after normalized to that of wt cells before starvation. Positions of substrates, GST-c-Jun(1–79) and MBP, are indicated.
Defective JNK Activation in btk-Mutated Mast Cells upon FcɛRI Crosslinking or Growth Factor Stimulation.
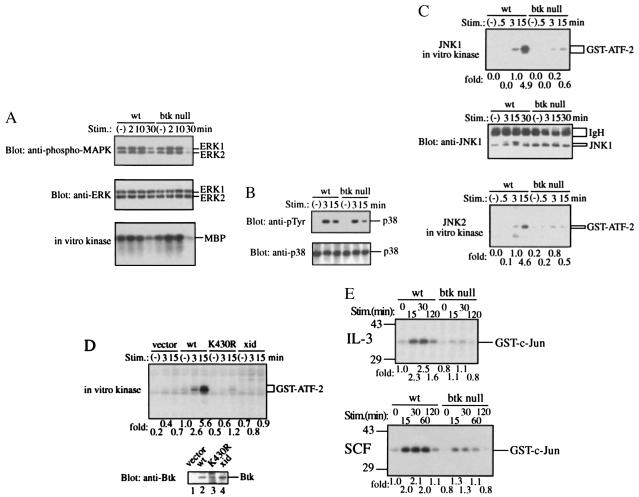
Activities of ERKs, JNKs, and p38 were measured in BMMCs prepared from wt and btk null mice before and after FcɛRI crosslinking. In addition to ERK1 and ERK2, the activation of which was previously shown (20, 21), JNK1, JNK2, and p38 were activated in wt-BMMC upon FcɛRI crosslinking (Fig. 4). Both ERK1 and ERK2 were activated equally well in btk null-BMMC (Fig. 4A), while p38 activity, as assessed by tyrosine phosphorylation, was slightly affected in btk null mast cells (Fig. 4B). In these cells tyrosine phosphorylation of p38 returned to the basal level earlier than that in wt mast cells. In contrast, the activities of JNK1 and JNK2 were drastically compromised in btk null-BMMC (Fig. 4C). As measured by immune complex kinase assays using GST-ATF-2 as a substrate, kinase activities of both JNK1 and JNK2 in btk null-BMMC were less than one tenth of those in wt-BMMC at its peak activity (15 min) (Fig. 4C). Essentially the same results were obtained when GST-c-Jun(1–79) was used as a substrate (data not shown). Similarly, BMMC derived from xid mice showed a reduced activation of JNK compared with CBA/J (wt)-BMMC upon FcɛRI crosslinking (data not shown).
Figure 4.
Defective JNK activation in btk null-BMMC upon FcɛRI crosslinking and stimulation with growth factor. (A) ERK phosphorylation and catalytic activity. BMMCs from B6/129 F2 (wt) and btk null mice were stimulated through FcɛRI for the indicated periods. ERK phosphorylation was assayed by immunoblotting with anti-phospho-MAPK (Top). The same blot was reprobed with anti-p44/42 MAPK antibody to confirm a comparable expression of ERK1/2 (Middle). ERK1 activity was measured by in vitro immune complex kinase assays using MBP as a substrate. Representative of three similar experiments. (B) Tyrosine phosphorylation of p38. BMMCs were stimulated as described above. p38 immunoprecipitates were analyzed by immunoblotting with anti-phosphotyrosine antibody. The same blot was reprobed with anti-p38. Representative of three similar experiments. (C) JNK activity. Kinase activity was measured on immune complexes precipitated with anti-JNK1 (Top) or anti-JNK2 (Bottom) using GST-ATF-2 as a substrate. -Fold activities measured by densitometry of the autoradiograms were normalized to that of wt cells at 3 min poststimulation. The blot onto which the JNK1 kinase reaction products were transferred was probed with anti-JNK1 (Middle). Of note is that the JNK1 band with a slower mobility due to phosphorylation was conspicuous with wt-BMMC stimulated for 3–15 min in contrast with the weaker intensity of the corresponding band in btk null-BMMC. Representative of three similar experiments. Detection of JNK2 bands by reprobing of the JNK2 kinase blots was hampered by the closeness of the molecular mass of JNK2 to that of immunoglobulin heavy chains. (D) Wt, K430R, and xid (R28C) btk retroviruses with the puromycin resistance gene were prepared using BOSC-23 packaging cells. Infected, puromycin-selected BMMCs were immunologically stimulated as described in Materials and Methods. JNK1 kinase activity was measured. -Fold activities were normalized to that of wt cells before stimulation. Lower shows the expression level of Btk in reconstituted cells as revealed by immunoblotting of total cell lysates by anti-Btk antibody. (E) Wt- and btk null-BMMCs were stimulated with recombinant mouse IL-3 or stem cell factor for the indicated times after an overnight starvation of growth factors. Then, JNK1 activity was measured. -Fold activities were normalized to that of wt cells before stimulation.
To confirm the specific effect of the btk mutations on the activation of JNK, btk null-BMMC was reconstituted with the expression vectors encoding wt, kinase-dead (K430R), or xid (R28C) Btk proteins. Upon FcɛRI crosslinking, btk null-BMMC reconstituted with the wt btk cDNA exhibited the JNK activation similar to that in wt-BMMC while JNK activation in btk null-BMMC reconstituted with the kinase-dead (K430R) btk was as low as that in btk null-BMMC transfected with the vector control (Fig. 4D). As expected from the xid phenotype, btk null-BMMC reconstituted with the btk cDNA with xid mutation showed defective JNK activation. p38 activation upon FcɛRI crosslinking was also restored in the wt btk, but not kinase-dead btk, transfectants (data not shown). Transfection of btk null-BMMC with syk or lyn cDNAs did not restore JNK activation, indicating that Btk is specifically involved in JNK activation induced by FcɛRI crosslinking (data not shown).
Furthermore, the ability of Btk to regulate JNK was shown when BMMCs deprived of growth factors overnight were stimulated with IL-3 or stem cell factor. Both growth factors induced stronger, transient activation of JNK1 in wt-BMMC compared with that in btk null-BMMC (Fig. 4E). Collectively, these data show that Btk regulates JNK activity under various conditions.
DISCUSSION
The present study demonstrates that Btk regulates apoptosis as well as kinase activities of JNK and p38. The intact kinase activity of Btk is required for both of the activities. The intactness of the pleckstrin homology domain of Btk was also required for growth factor deprivation-induced apoptosis, since expression of the Btk protein with xid mutation (R28C) in the pleckstrin homology domain failed to respond by rapid apoptotic cell death. This is in a sharp contrast with radiation-induced apoptosis in DT-40 chicken lymphoma B cells, where xid mutated Btk induced apoptosis as well as wt Btk did (22).
Increased ceramide levels generated by various stresses induced apoptosis in a JNK-dependent manner (19), and apoptosis of sympathetic neurons after nerve growth factor deprivation is inhibited by a c-Jun dominant negative mutant (23). These previous studies as well as our present study indicate that apoptotic cell death induced by growth factor deprivation is dependent on JNK activity. However, it is noteworthy that the transient activation of JNK does not seem to lead to apoptosis. Thus, either growth factor stimulation or FcɛRI crosslinking did not induce apoptosis but rather induce DNA synthesis (data not shown). Activation of ERKs induced by these stimuli may oppose JNK activation to suppress apoptosis, as proposed for apoptosis of PC12 cells deprived of nerve growth factor (18). JNK was also implicated in cell growth (24) and transformation (25). However, the mechanism by which Btk regulates JNK is currently unknown. Since Btk is activated rapidly by stimulation of various cell surface receptors, including B cell antigen receptor (26, 27) and FcɛRI (8), and since it can be activated by Src family kinases (9, 10), it probably works upstream of Ras or Rho family GTPases. Activated Cdc42 and Rac eventually lead to activation of JNK through the phosphorylation cascade, PAK65 → MEKK → SEK1 → JNK. Indeed, PAK65 activity in wt mast cells upon FcɛRI crosslinking was higher than in btk-mutated mast cells, and FcɛRI-induced JNK activation was at least partially inhibited by a dominant negative SEK1(Lys → Arg) (data not shown). Therefore, Btk appears to regulate JNK through this cascade. The known JNK phosphorylation targets include c-Jun and ATF-2 (24, 28–30). These transcription factors regulate the expression of multiple cytokine genes, including tumor necrosis factor α gene (31). Consistent with the defective JNK activation, xid- or btk null-BMMCs showed a severe defect in FcɛRI-induced secretion of this and other cytokines (D.H., Y.K., Naoki Inagaki, T. Kitamura, W.N.K., M.M.-Y., T.M., W. Han, S.E.H., L.Y., H. Nagai, A. E. Goldfeld, F.W.A., O. N. Witte, and T. Kawakami, unpublished data). Although more subtly affected in btk mutated cells, the activity of p38 also seems to be regulated by Btk. To our knowledge, the regulation of apoptosis is the first known biological function of Btk in mast cells. This function appears to be controlled through the regulation of JNK/p38 activity. Studies on mechanisms for JNK/p38 activation by Btk are under way.
Acknowledgments
We thank Drs. D. Baltimore and J. Han for kind gifts of reagents, Drs. A. Altman and D. Green for critical reading of the manuscript, and Artin Mahboubi for help in DNA analysis. This work was supported in part by National Institutes of Health Grants RO1 AI33617 and RO1 AI38348 (T.K.). This is publication 160 from the La Jolla Institute for Allergy and Immunology.
ABBREVIATIONS
- Btk
Bruton’s tyrosine kinase
- BMMC
bone marrow-derived mouse mast cells
- MAPK
mitogen-activated protein kinase
- MBP
myelin basic protein
- ERK
extracellular signal-regulated kinase
- FcɛRI
high-affinity IgE receptor
- IL
interleukin
- JNK
c-Jun N terminal kinase or stress-activated protein kinase
- wt
wild-type
- GST
glutathione S-transferase
References
- 1.Yamada N, Kawakami Y, Kimura H, Fukamachi H, Baier G, Altman A, Kato T, Inagaki Y, Kawakami T. Biochem Biophys Res Commun. 1993;192:231–240. doi: 10.1006/bbrc.1993.1404. [DOI] [PubMed] [Google Scholar]
- 2.Vetrie D, Vorechovsky I, Sideras P, Holland J, Davies A, Flinter F, Hammarstrom L, Kinnon C, Levinsky R, Bobrow M, Smith C I E, Bentley D R. Nature (London) 1993;361:226–233. doi: 10.1038/361226a0. [DOI] [PubMed] [Google Scholar]
- 3.Tsukada S, Saffran D, Rawlings D J, Parolini O, Allen R C, Klisak I, Sparkes R S, Kubagawa H, Mohandas T, Quan S, Belmont J W, Cooper M D, Conley M E, Witte O N. Cell. 1993;72:279–290. doi: 10.1016/0092-8674(93)90667-f. [DOI] [PubMed] [Google Scholar]
- 4.Thomas J D, Sideras P, Smith C I E, Vorechovsky I, Chapman V, Paul W E. Science. 1993;261:355–358. doi: 10.1126/science.8332900. [DOI] [PubMed] [Google Scholar]
- 5.Rawlings D J, Saffran D C, Tsukada S, Largaespada D A, Grimaldi J C, Cohen L, Mohr R N, Bazan J F, Howard M, Copeland N G, Jenkins N A, Witte O N. Science. 1993;261:358–361. doi: 10.1126/science.8332901. [DOI] [PubMed] [Google Scholar]
- 6.Khan W N, Alt F W, Gerstein R M, Malynn B A, Larsson I, Rathbun G, Davidson L, Mueller S, Kantor A B, Herzenberg L A, Rosen F S, Sideras P. Immunity. 1995;3:283–299. doi: 10.1016/1074-7613(95)90114-0. [DOI] [PubMed] [Google Scholar]
- 7.Kerner J D, Appleby M W, Mohr R N, Chien S, Rawlings D J, Maliszewski C R, Witte O N, Perlmutter R M. Immunity. 1995;3:301–312. doi: 10.1016/1074-7613(95)90115-9. [DOI] [PubMed] [Google Scholar]
- 8.Kawakami Y, Yao L, Tsukada S, Witte O N, Kawakami T. Mol Cell Biol. 1994;14:5108–5113. doi: 10.1128/mcb.14.8.5108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rawlings D J, Scharenberg A M, Park H, Wahl M I, Lin S, Kato R M, Fluckiger A-C, Witte O N. Science. 1996;271:822–825. doi: 10.1126/science.271.5250.822. [DOI] [PubMed] [Google Scholar]
- 10.Mahajan S, Fargnoli J, Burkhardt A L, Kut S A, Saouaf S J, Bolen J B. Mol Cell Biol. 1995;15:5304–5311. doi: 10.1128/mcb.15.10.5304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park H, Wahl M I, Afar D E H, Turck C W, Rawlings D J, Tam C, Scharenberg A M, Kinet J-P, Witte O N. Immunity. 1996;4:515–525. doi: 10.1016/s1074-7613(00)80417-3. [DOI] [PubMed] [Google Scholar]
- 12.Yao L, Kawakami Y, Kawakami T. Proc Natl Acad Sci USA. 1994;91:9175–9179. doi: 10.1073/pnas.91.19.9175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takata M, Kurosaki T. J Exp Med. 1996;184:31–40. doi: 10.1084/jem.184.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kawakami T, Inagaki N, Takei M, Fukamachi H, Coggeshall K M, Ishizaka K, Ishizaka T. J Immunol. 1992;148:3513–3519. [PubMed] [Google Scholar]
- 15.Onishi M, Kinoshita S, Morikawa Y, Shibuya A, Phillips J, Lanier L L, Gorman D M, Nolan G P, Miyajima A, Kitamura T. Exp Hematol. 1996;24:324–329. [PubMed] [Google Scholar]
- 16.Pear W S, Nolan G P, Scott M L, Baltimore D. Proc Natl Acad Sci USA. 1993;90:8392–8396. doi: 10.1073/pnas.90.18.8392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mekori Y A, Oh C K, Metcalfe D D. J Immunol. 1993;151:3775–3784. [PubMed] [Google Scholar]
- 18.Xia Z, Dickens M, Raingeaud J, Davis R J, Greenberg M E. Science. 1995;270:1326–1331. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]
- 19.Verheij M, Bose R, Lin X H, Yao B, Jarvis W D, Grant S, Birrer M J, Szabo E, Zon L I, Kyriakis J M, Haimovitz-Friedman A, Fuks Z, Kolesnick R N. Nature (London) 1996;380:75–79. doi: 10.1038/380075a0. [DOI] [PubMed] [Google Scholar]
- 20.Fukamachi H, Takei M, Kawakami T. Int Arch Allergy Immunol. 1993;102:15–25. doi: 10.1159/000236546. [DOI] [PubMed] [Google Scholar]
- 21.Tsai M, Chen R-H, Tam S-Y, Blenis J, Galli S J. Eur J Immunol. 1993;23:3286–3291. doi: 10.1002/eji.1830231234. [DOI] [PubMed] [Google Scholar]
- 22.Uckun F M, Waddick K G, Mahajan S, Jun X, Takata M, Bolen J, Kurosaki T. Science. 1996;273:1096–1100. doi: 10.1126/science.273.5278.1096. [DOI] [PubMed] [Google Scholar]
- 23.Ham J, Babij C, Whitfield J, Pfarr C M, Lallemand D, Yaniv M, Rubin L L. Neuron. 1995;14:927–939. doi: 10.1016/0896-6273(95)90331-3. [DOI] [PubMed] [Google Scholar]
- 24.Derijard B, Hibi M, Wu I-H, Barrett T, Su B, Deng T, Karin M, Davis R J. Cell. 1994;76:1025–1037. doi: 10.1016/0092-8674(94)90380-8. [DOI] [PubMed] [Google Scholar]
- 25.Raitano A B, Halpern J R, Hambuch T M, Sawyers C L. Proc Natl Acad Sci USA. 1995;92:11746–11750. doi: 10.1073/pnas.92.25.11746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saouaf S J, Mahajan S, Rowley R B, Kut S A, Fargnoli J, Burkhardt A L, Tsukada S, Witte O N, Bolen J B. Proc Natl Acad Sci USA. 1994;91:9524–9528. doi: 10.1073/pnas.91.20.9524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aoki Y, Isselbacher K J, Pillai S. Proc Natl Acad Sci USA. 1994;91:10606–10609. doi: 10.1073/pnas.91.22.10606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gupta S, Campbell D, Derijard B, Davis R J. Science. 1995;267:389–393. doi: 10.1126/science.7824938. [DOI] [PubMed] [Google Scholar]
- 29.Livingstone C, Patel G, Jones N. EMBO J. 1995;14:1785–1797. doi: 10.1002/j.1460-2075.1995.tb07167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Dam H, Wilhelm D, Herr I, Steffen A, Herrlich P, Angel P. EMBO J. 1995;14:1798–1811. doi: 10.1002/j.1460-2075.1995.tb07168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tsai E Y, Jain J, Pesavento P A, Rao A, Goldfeld A E. Mol Cell Biol. 1996;16:459–467. doi: 10.1128/mcb.16.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]