Abstract
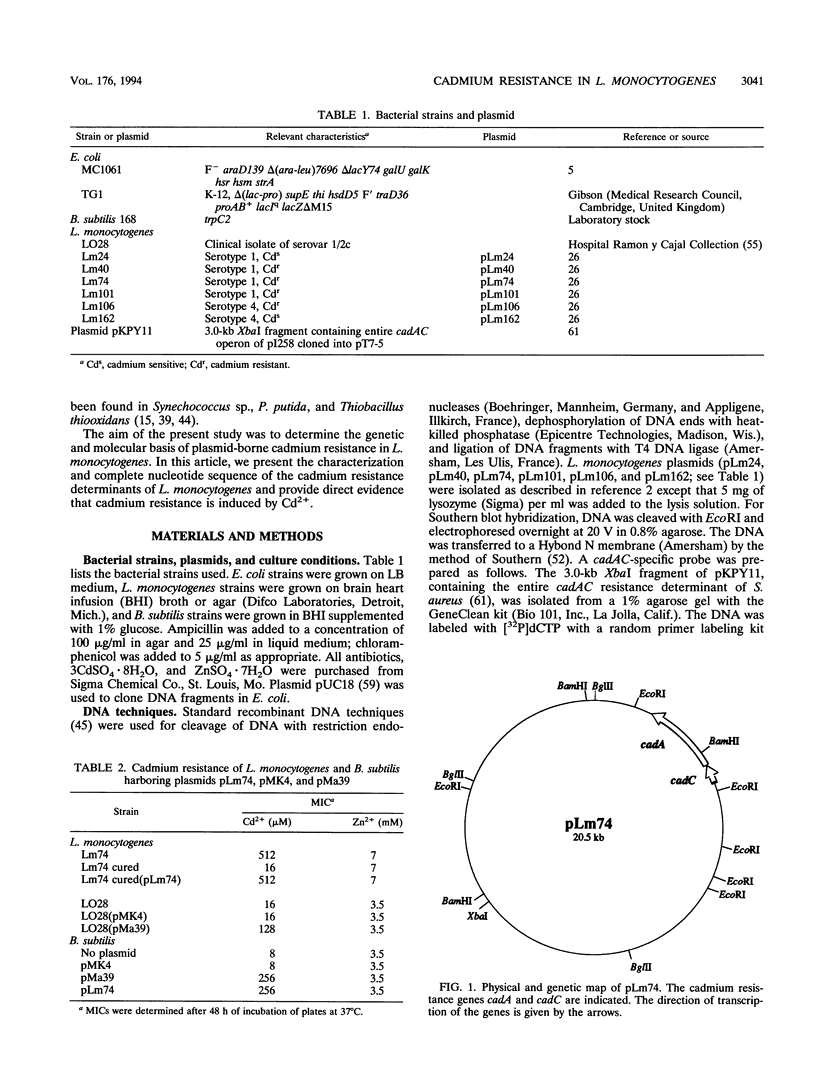
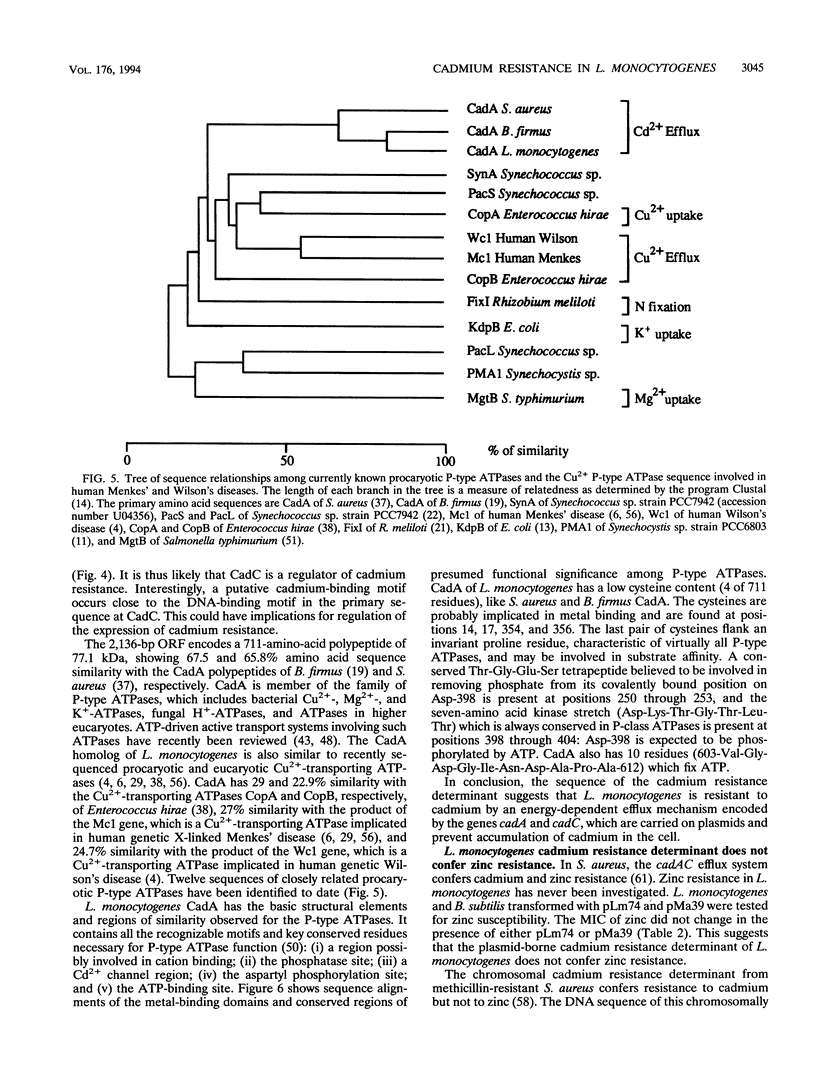
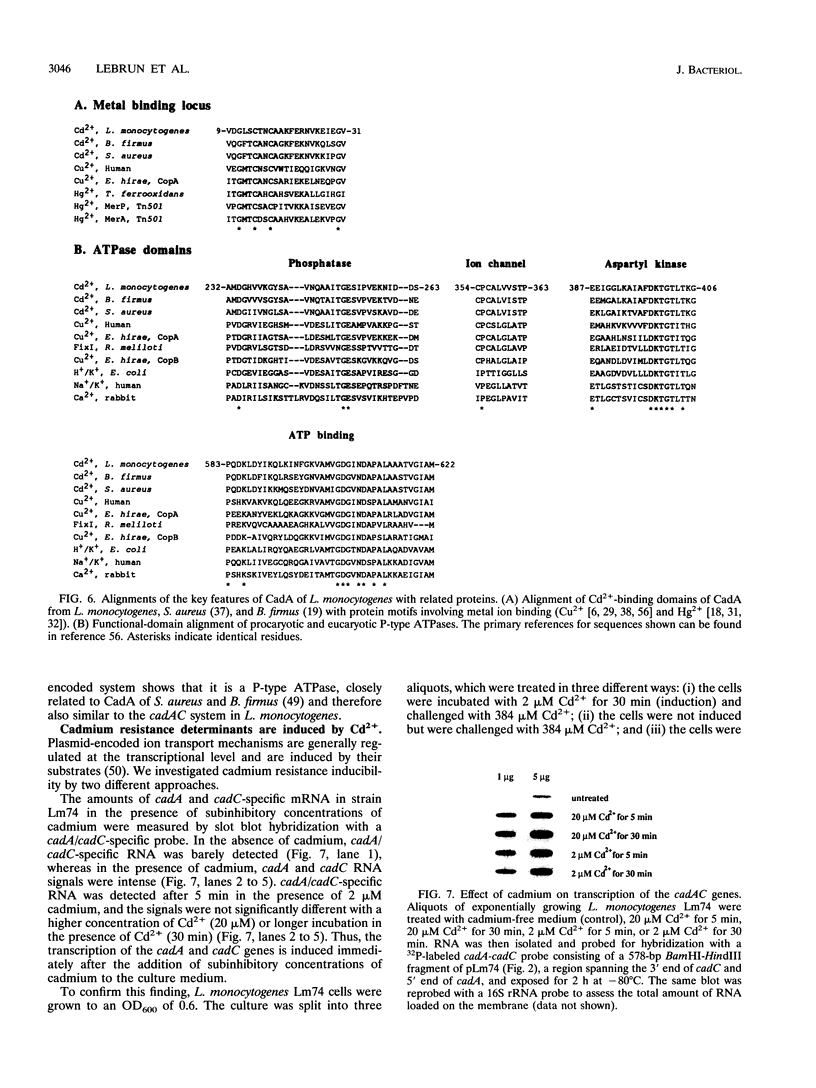
pLm74 is the smallest known plasmid in Listeria monocytogenes. It confers resistance to the toxic divalent cation cadmium. It contains a 3.1-kb EcoRI fragment which hybridizes with the cadAC genes of plasmid pI258 of Staphylococcus aureus. When introduced into cadmium-sensitive L. monocytogenes or Bacillus subtilis strains, this fragment conferred cadmium resistance. The DNA sequence of the 3.1-kb EcoRI fragment contains two open reading frames, cadA and cadC. The deduced amino acid sequences are similar to those of the cad operon of plasmid pI258 of S. aureus, known to prevent accumulation of Cd2+ in the bacteria by an ATPase efflux mechanism. The cadmium resistance determinant of L. monocytogenes does not confer zinc resistance, in contrast to the cadAC determinant of S. aureus, suggesting that the two resistance mechanisms are slightly different. Slot blot DNA-RNA hybridization analysis showed cadmium-inducible synthesis of L. monocytogenes cadAC RNA.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altschul S. F., Gish W., Miller W., Myers E. W., Lipman D. J. Basic local alignment search tool. J Mol Biol. 1990 Oct 5;215(3):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borer P. N., Dengler B., Tinoco I., Jr, Uhlenbeck O. C. Stability of ribonucleic acid double-stranded helices. J Mol Biol. 1974 Jul 15;86(4):843–853. doi: 10.1016/0022-2836(74)90357-x. [DOI] [PubMed] [Google Scholar]
- Bull P. C., Thomas G. R., Rommens J. M., Forbes J. R., Cox D. W. The Wilson disease gene is a putative copper transporting P-type ATPase similar to the Menkes gene. Nat Genet. 1993 Dec;5(4):327–337. doi: 10.1038/ng1293-327. [DOI] [PubMed] [Google Scholar]
- Casadaban M. J., Cohen S. N. Analysis of gene control signals by DNA fusion and cloning in Escherichia coli. J Mol Biol. 1980 Apr;138(2):179–207. doi: 10.1016/0022-2836(80)90283-1. [DOI] [PubMed] [Google Scholar]
- Chelly J., Tümer Z., Tønnesen T., Petterson A., Ishikawa-Brush Y., Tommerup N., Horn N., Monaco A. P. Isolation of a candidate gene for Menkes disease that encodes a potential heavy metal binding protein. Nat Genet. 1993 Jan;3(1):14–19. doi: 10.1038/ng0193-14. [DOI] [PubMed] [Google Scholar]
- El Solh N., Ehrlich S. D. A small cadmium resistance plasmid isolated from Staphylococcus aureus. Plasmid. 1982 Jan;7(1):77–84. doi: 10.1016/0147-619x(82)90029-4. [DOI] [PubMed] [Google Scholar]
- Farber J. M., Peterkin P. I. Listeria monocytogenes, a food-borne pathogen. Microbiol Rev. 1991 Sep;55(3):476–511. doi: 10.1128/mr.55.3.476-511.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaillard J. L., Berche P., Frehel C., Gouin E., Cossart P. Entry of L. monocytogenes into cells is mediated by internalin, a repeat protein reminiscent of surface antigens from gram-positive cocci. Cell. 1991 Jun 28;65(7):1127–1141. doi: 10.1016/0092-8674(91)90009-n. [DOI] [PubMed] [Google Scholar]
- Geisler M., Richter J., Schumann J. Molecular cloning of a P-type ATPase gene from the cyanobacterium Synechocystis sp. PCC 6803. Homology to eukaryotic Ca(2+)-ATPases. J Mol Biol. 1993 Dec 20;234(4):1284–1289. doi: 10.1006/jmbi.1993.1684. [DOI] [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene. 1984 Jun;28(3):351–359. doi: 10.1016/0378-1119(84)90153-7. [DOI] [PubMed] [Google Scholar]
- Hesse J. E., Wieczorek L., Altendorf K., Reicin A. S., Dorus E., Epstein W. Sequence homology between two membrane transport ATPases, the Kdp-ATPase of Escherichia coli and the Ca2+-ATPase of sarcoplasmic reticulum. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4746–4750. doi: 10.1073/pnas.81.15.4746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins D. G., Bleasby A. J., Fuchs R. CLUSTAL V: improved software for multiple sequence alignment. Comput Appl Biosci. 1992 Apr;8(2):189–191. doi: 10.1093/bioinformatics/8.2.189. [DOI] [PubMed] [Google Scholar]
- Higham D. P., Sadler P. J., Scawen M. D. Cadmium-Resistant Pseudomonas putida Synthesizes Novel Cadmium Proteins. Science. 1984 Sep 7;225(4666):1043–1046. doi: 10.1126/science.225.4666.1043. [DOI] [PubMed] [Google Scholar]
- Horitsu H., Yamamoto K., Wachi S., Kawai K., Fukuchi A. Plasmid-determined cadmium resistance in Pseudomonas putida GAM-1 isolated from soil. J Bacteriol. 1986 Jan;165(1):334–335. doi: 10.1128/jb.165.1.334-335.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huckle J. W., Morby A. P., Turner J. S., Robinson N. J. Isolation of a prokaryotic metallothionein locus and analysis of transcriptional control by trace metal ions. Mol Microbiol. 1993 Jan;7(2):177–187. doi: 10.1111/j.1365-2958.1993.tb01109.x. [DOI] [PubMed] [Google Scholar]
- Inoue C., Sugawara K., Kusano T. Thiobacillus ferrooxidans mer operon: sequence analysis of the promoter and adjacent genes. Gene. 1990 Nov 30;96(1):115–120. doi: 10.1016/0378-1119(90)90349-v. [DOI] [PubMed] [Google Scholar]
- Ivey D. M., Guffanti A. A., Shen Z., Kudyan N., Krulwich T. A. The cadC gene product of alkaliphilic Bacillus firmus OF4 partially restores Na+ resistance to an Escherichia coli strain lacking an Na+/H+ antiporter (NhaA). J Bacteriol. 1992 Aug;174(15):4878–4884. doi: 10.1128/jb.174.15.4878-4884.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji G., Silver S. Regulation and expression of the arsenic resistance operon from Staphylococcus aureus plasmid pI258. J Bacteriol. 1992 Jun;174(11):3684–3694. doi: 10.1128/jb.174.11.3684-3694.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn D., David M., Domergue O., Daveran M. L., Ghai J., Hirsch P. R., Batut J. Rhizobium meliloti fixGHI sequence predicts involvement of a specific cation pump in symbiotic nitrogen fixation. J Bacteriol. 1989 Feb;171(2):929–939. doi: 10.1128/jb.171.2.929-939.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondorosi E., Pierre M., Cren M., Haumann U., Buiré M., Hoffmann B., Schell J., Kondorosi A. Identification of NolR, a negative transacting factor controlling the nod regulon in Rhizobium meliloti. J Mol Biol. 1991 Dec 20;222(4):885–896. doi: 10.1016/0022-2836(91)90583-r. [DOI] [PubMed] [Google Scholar]
- Lebrun M., Audurier A., Cossart P. Plasmid-borne cadmium resistance genes in Listeria monocytogenes are present on Tn5422, a novel transposon closely related to Tn917. J Bacteriol. 1994 May;176(10):3049–3061. doi: 10.1128/jb.176.10.3049-3061.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebrun M., Loulergue J., Chaslus-Dancla E., Audurier A. Plasmids in Listeria monocytogenes in relation to cadmium resistance. Appl Environ Microbiol. 1992 Sep;58(9):3183–3186. doi: 10.1128/aem.58.9.3183-3186.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mengaud J., Geoffroy C., Cossart P. Identification of a new operon involved in Listeria monocytogenes virulence: its first gene encodes a protein homologous to bacterial metalloproteases. Infect Immun. 1991 Mar;59(3):1043–1049. doi: 10.1128/iai.59.3.1043-1049.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer J. F., Livingston J., Hall B., Paynter J. A., Begy C., Chandrasekharappa S., Lockhart P., Grimes A., Bhave M., Siemieniak D. Isolation of a partial candidate gene for Menkes disease by positional cloning. Nat Genet. 1993 Jan;3(1):20–25. doi: 10.1038/ng0193-20. [DOI] [PubMed] [Google Scholar]
- Michel E., Reich K. A., Favier R., Berche P., Cossart P. Attenuated mutants of the intracellular bacterium Listeria monocytogenes obtained by single amino acid substitutions in listeriolysin O. Mol Microbiol. 1990 Dec;4(12):2167–2178. doi: 10.1111/j.1365-2958.1990.tb00578.x. [DOI] [PubMed] [Google Scholar]
- Misra T. K., Brown N. L., Fritzinger D. C., Pridmore R. D., Barnes W. M., Haberstroh L., Silver S. Mercuric ion-resistance operons of plasmid R100 and transposon Tn501: the beginning of the operon including the regulatory region and the first two structural genes. Proc Natl Acad Sci U S A. 1984 Oct;81(19):5975–5979. doi: 10.1073/pnas.81.19.5975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra T. K., Brown N. L., Haberstroh L., Schmidt A., Goddette D., Silver S. Mercuric reductase structural genes from plasmid R100 and transposon Tn501: functional domains of the enzyme. Gene. 1985;34(2-3):253–262. doi: 10.1016/0378-1119(85)90134-9. [DOI] [PubMed] [Google Scholar]
- Nies D. H. CzcR and CzcD, gene products affecting regulation of resistance to cobalt, zinc, and cadmium (czc system) in Alcaligenes eutrophus. J Bacteriol. 1992 Dec;174(24):8102–8110. doi: 10.1128/jb.174.24.8102-8110.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nies D. H., Nies A., Chu L., Silver S. Expression and nucleotide sequence of a plasmid-determined divalent cation efflux system from Alcaligenes eutrophus. Proc Natl Acad Sci U S A. 1989 Oct;86(19):7351–7355. doi: 10.1073/pnas.86.19.7351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nies D. H., Silver S. Plasmid-determined inducible efflux is responsible for resistance to cadmium, zinc, and cobalt in Alcaligenes eutrophus. J Bacteriol. 1989 Feb;171(2):896–900. doi: 10.1128/jb.171.2.896-900.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick R. P., Murphy E., Gryczan T. J., Baron E., Edelman I. Penicillinase plasmids of Staphylococcus aureus: restriction-deletion maps. Plasmid. 1979 Jan;2(1):109–129. doi: 10.1016/0147-619x(79)90010-6. [DOI] [PubMed] [Google Scholar]
- Nucifora G., Chu L., Misra T. K., Silver S. Cadmium resistance from Staphylococcus aureus plasmid pI258 cadA gene results from a cadmium-efflux ATPase. Proc Natl Acad Sci U S A. 1989 May;86(10):3544–3548. doi: 10.1073/pnas.86.10.3544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odermatt A., Suter H., Krapf R., Solioz M. Primary structure of two P-type ATPases involved in copper homeostasis in Enterococcus hirae. J Biol Chem. 1993 Jun 15;268(17):12775–12779. [PubMed] [Google Scholar]
- Olafson R. W., McCubbin W. D., Kay C. M. Primary- and secondary-structural analysis of a unique prokaryotic metallothionein from a Synechococcus sp. cyanobacterium. Biochem J. 1988 May 1;251(3):691–699. doi: 10.1042/bj2510691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry R. D., Silver S. Cadmium and manganese transport in Staphylococcus aureus membrane vesicles. J Bacteriol. 1982 May;150(2):973–976. doi: 10.1128/jb.150.2.973-976.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenstein R., Peschel A., Wieland B., Götz F. Expression and regulation of the antimonite, arsenite, and arsenate resistance operon of Staphylococcus xylosus plasmid pSX267. J Bacteriol. 1992 Jun;174(11):3676–3683. doi: 10.1128/jb.174.11.3676-3683.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- San Francisco M. J., Hope C. L., Owolabi J. B., Tisa L. S., Rosen B. P. Identification of the metalloregulatory element of the plasmid-encoded arsenical resistance operon. Nucleic Acids Res. 1990 Feb 11;18(3):619–624. doi: 10.1093/nar/18.3.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver S., Nucifora G., Chu L., Misra T. K. Bacterial resistance ATPases: primary pumps for exporting toxic cations and anions. Trends Biochem Sci. 1989 Feb;14(2):76–80. doi: 10.1016/0968-0004(89)90048-0. [DOI] [PubMed] [Google Scholar]
- Silver S., Nucifora G., Phung L. T. Human Menkes X-chromosome disease and the staphylococcal cadmium-resistance ATPase: a remarkable similarity in protein sequences. Mol Microbiol. 1993 Oct;10(1):7–12. doi: 10.1111/j.1365-2958.1993.tb00898.x. [DOI] [PubMed] [Google Scholar]
- Silver S., Walderhaug M. Gene regulation of plasmid- and chromosome-determined inorganic ion transport in bacteria. Microbiol Rev. 1992 Mar;56(1):195–228. doi: 10.1128/mr.56.1.195-228.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snavely M. D., Miller C. G., Maguire M. E. The mgtB Mg2+ transport locus of Salmonella typhimurium encodes a P-type ATPase. J Biol Chem. 1991 Jan 15;266(2):815–823. [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Sullivan M. A., Yasbin R. E., Young F. E. New shuttle vectors for Bacillus subtilis and Escherichia coli which allow rapid detection of inserted fragments. Gene. 1984 Jul-Aug;29(1-2):21–26. doi: 10.1016/0378-1119(84)90161-6. [DOI] [PubMed] [Google Scholar]
- Tanaka M., Kawahara Y., Motomiya T., Sakamoto M., Sugiura M., Toyoda M., Kajita A., Osamura Y. Cardiomyopathy characterized by abnormal accumulation of desmin-type intermediate filaments in cardiac muscle fibers. A case report and review of the literature. Acta Pathol Jpn. 1989 Apr;39(4):266–273. doi: 10.1111/j.1440-1827.1989.tb01511.x. [DOI] [PubMed] [Google Scholar]
- Vallee B. L., Ulmer D. D. Biochemical effects of mercury, cadmium, and lead. Annu Rev Biochem. 1972;41(10):91–128. doi: 10.1146/annurev.bi.41.070172.000515. [DOI] [PubMed] [Google Scholar]
- Vulpe C., Levinson B., Whitney S., Packman S., Gitschier J. Isolation of a candidate gene for Menkes disease and evidence that it encodes a copper-transporting ATPase. Nat Genet. 1993 Jan;3(1):7–13. doi: 10.1038/ng0193-7. [DOI] [PubMed] [Google Scholar]
- Williams S. G., Attridge S. R., Manning P. A. The transcriptional activator HlyU of Vibrio cholerae: nucleotide sequence and role in virulence gene expression. Mol Microbiol. 1993 Aug;9(4):751–760. doi: 10.1111/j.1365-2958.1993.tb01735.x. [DOI] [PubMed] [Google Scholar]
- Witte W., Green L., Misra T. K., Silver S. Resistance to mercury and to cadmium in chromosomally resistant Staphylococcus aureus. Antimicrob Agents Chemother. 1986 Apr;29(4):663–669. doi: 10.1128/aac.29.4.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Yoon K. P., Misra T. K., Silver S. Regulation of the cadA cadmium resistance determinant of Staphylococcus aureus plasmid pI258. J Bacteriol. 1991 Dec;173(23):7643–7649. doi: 10.1128/jb.173.23.7643-7649.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon K. P., Silver S. A second gene in the Staphylococcus aureus cadA cadmium resistance determinant of plasmid pI258. J Bacteriol. 1991 Dec;173(23):7636–7642. doi: 10.1128/jb.173.23.7636-7642.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]