Abstract
Binding of CD95 (Fas/APO-1) by its ligand (CD95L) commonly induces apoptosis. Apoptosis of activated T cells, induced by CD95L expressed in the rodent testis, has been proposed to be the mechanism of immune privilege [Bellgrau, D., Gold, D., Selawry, H., Moore, J., Franzusoff, A. & Duke, R. C. (1995) Nature (London) 377, 630–632]. To test whether CD95L could protect pancreatic islet grafts from rejection, we made transgenic mice expressing murine CD95L on their islet β cells and transplanted fetal pancreata under the kidney capsules of allogeneic animals. Expression of CD95L failed to protect the grafts from rejection. However, transgenic mice developed a granulocytic infiltration in their pancreata. These results demonstrate a pro-inflammatory function of CD95L and suggest that expression of CD95L may not be sufficient to protect organ allografts.
There is a chronic shortage of human tissues for transplantation that would be relieved if methods that allowed humans to accept grafts of xenogeneic tissue were developed. One of the main barriers to acceptance of xenogeneic and allogeneic grafts is the T cell-mediated response of the host. Immunosuppressant drugs that depress T cell activity leave the host vulnerable to infectious attack. A strategy allowing specific deletion of only those T cells that recognized the graft would allow tolerance of the graft without general immunosuppression.
It has been proposed (1, 2) that CD95 ligand (CD95L) may be able to function as just such a graft-specific immunosuppressant for three reasons. First, the testis is known as an immune-privileged site as foreign tissues grafted into the testis, in which the Sertoli cells express CD95L, persist for longer periods than when transplanted elsewhere (3). Second, activated T lymphocytes are known to express CD95 (reviewed in ref. 4) and become sensitive to CD95-mediated killing at some time following exposure to antigen (5). Third, it has been reported that testis grafts from normal mice, but not CD95L-mutant gld mice, survive for extended periods in allogeneic hosts (1). These observations suggested that CD95L expressed by Sertoli cells in the testis induced apoptosis of activated T cells that would otherwise mediate graft rejection. Furthermore, it raised the possibility that enforced expression of CD95L might be used to confer immune privilege on other cell types (6).
MATERIALS AND METHODS
Mice.
BALB/c, C57BL/6Jax (B6), C3H/HeJ (C3H), and C3H/HeJgld (C3Hgld) mice were bred at The Walter and Eliza Hall Institute for medical Research. Rat insulin promoter (RIP)–CD95L transgenic mice were made by injecting a DNA construct consisting of the rat insulin II promoter (7) linked to the murine CD95L cDNA (8), and simian virus 40 poly(A) addition sequences, into fertilized B6 oocytes. Transgenic animals were identified by Southern hybridization of tail DNA using a RIP probe.
Cell Death Assays.
Adult islets were isolated by digestion with collagenase P (Boehringer Mannheim) at 0.8 mg/ml, purified on a BSA gradient (First Link, Brierly Hill, U.K.) as described (9), and then hand-picked 2-fold and dispersed with trypsin. About 60–70% of this preparation comprised β cells as determined by flow cytometry (data not shown). Islet cells were cultured overnight in DMEM/10% fetal calf serum (FCS) to allow reexpression of CD95L in case it had been damaged by the isolation procedure. The next day, SKW6 B lymphoblastoid cells were cocultured for 24 hr with control or transgenic islet cells at an approximate ratio of 10 or 20:1 (105 SKW6 cells/104 islet cells). CD95–Fcγ fusion protein (20 μg/ml) or tumor necrosis factor receptor–Fcγ fusion protein (20 μg/ml; data not shown) were added to cultures to block their respective ligands (10). As a positive control for CD95-induced apoptosis, SKW6 cells were treated with APO-1 antibody (11). Cell viability was determined after 24 hr by visual inspection because it was easy to differentiate the large islet cells from the small SKW6 cells under phase contrast microscopy.
Grafting.
Testis tissue from 6- to 8-week-old donors or pancreas from fetal (embryonic days 16–18) donors were grafted under the kidney capsule of 6- to 8-week-old male recipients. Fetal pancreata were cultured for 2 weeks in vitro to allow degeneration of the exocrine pancreas (12). Expression of CD95L on β cells of the cultured pancreata was determined by immunocytochemistry. Freshly cut testis slices (approximately 2 × 2 × 2 mm) were grafted directly. Recovered grafts were fixed in Bouin’s solution. Sections were coded and the pathology was scored by an independent observer.
Histochemistry.
Preparation and insulin staining of Bouin’s solution-fixed or acetone-fixed cryostat sections were as described (13). Rat mAbs to Mac-1α/CD11b (M1/70) and Gr-1 (RB6–8C5) were detected with fluorescein isothiocyanate-coupled anti-rat Ig (Vector Laboratories) or horseradish peroxidase (HRP)-coupled anti-rat Ig (Chemicon). A rabbit anti-mouse CD95L polyclonal antibody (AL82; ref. 14) was diluted 1:100 and detected with HRP-coupled anti-rabbit Ig (Dako) or fluorescein isothiocyanate-coupled anti-rabbit Ig (Silenus, Paris). Blocking using 2% FCS plus 10% normal mouse serum was done before staining with the rat or rabbit antibodies. Peroxidase staining was developed with diaminobenzidine, and sections were counterstained with hematoxylin.
RESULTS
CD95L Transgenic Mice.
To test whether CD95L could protect allogeneic islet grafts, we made transgenic mice expressing the murine CD95L cDNA (8) driven by the rat insulin II promoter (7) (RIP–CD95L mice). Two lines were investigated, one of which expressed a higher level of CD95L (RIP–CD95Lhi line) detectable by a rabbit polyclonal antibody (14) that showed a similar level of staining to Sertoli cells in the testis (Fig. 1 A and B). The other transgenic mice (RIP–CD95Llo line) expressed a lower level of CD95L which did not stain with the antibody by immunocytochemistry. Nevertheless, adult β cells from both lines expressed sufficient functional CD95L to give an apoptotic signal as islets from transgenic mice, but not those from control littermates, killed SKW6 B lymphoblastoid cells, which are susceptible to CD95-mediated apoptosis, in tissue culture (Fig. 2). Killing in this assay depended on CD95–CD95L interactions as it could be inhibited by CD95–Fcγ fusion protein that binds to and blocks CD95L (10). Killing was not inhibited by a control tumor necrosis factor receptor–Fcγ fusion protein that blocks tumor necrosis factor receptors (data not shown).
Figure 1.
Expression of CD95L by transgenic islet β cells (A) and by Sertoli cells in the testis (B). Cryostat sections from a RIP–CD95Lhi pancreas or a normal testis were fixed in acetone and then stained for CD95L using a rabbit polyclonal antibody detected with an fluorescein isothiocyanate-coupled antibody to rabbit Ig. The same tissues were stained with an irrelevant rabbit polyclonal antibody (C and D).
Figure 2.
Killing of SKW6 B lymphoblastoid cells by anti-CD95 antibody or by RIP–CD95L+ adult islet β cells. SKW6 B cells are killed by increasing concentrations of anti-CD95 antibody (left side of graph) or by incubation with RIP–CD95L+ adult islet cells expressing different amounts of transgenic CD95L (right side of graph). A CD95–Fcγ fusion protein that binds to and blocks CD95L was added where indicated. Results shown are averages of three experiments performed on separate occasions. Error bars indicate 2 × SEM.
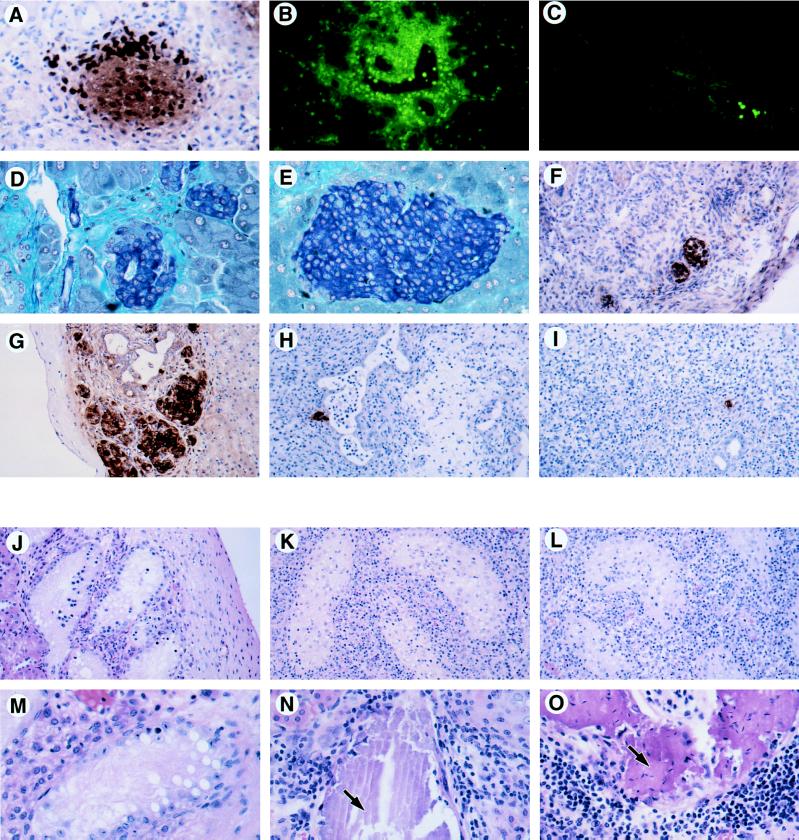
Most islets of young (9–12 days) RIP–CD95Lhi transgenic mice were infiltrated by large numbers of neutrophils, eosinophils, and macrophages, but not by lymphocytes (Fig. 3A). In older (8-week-old) mice, occasional foci of Gr-1+ granulocytes, macrophages, and a few CD4+ T cells were evident (Fig. 3B), and most of the transgenic islets were small and disorganized, with associated ductal fibrosis (Fig. 3D). No such pathology was seen in littermate controls (Fig. 3 C and E). Young RIP–CD95Llo transgenic mice had a similar, but milder, pathology, with most of the islets from adults appearing normal (data not shown).
Figure 3.
Pathology of in situ and transplanted CD95L+ tissue. (A) Insulin staining by immunoperoxidase (brown cells) of a RIP–CD95Lhi islet from a 12-day-old mouse pancreas. The very dark stained cells are granulocytes (neutrophils and eosinophils) expressing endogenous peroxidase. (×200.) (B and C) Gr-1 staining (green cells) of a RIP–CD95Lhi 8-week-old pancreas (B) and a nontransgenic control pancreas (C). Gr-1+ neutrophils infiltrate the transgenic pancreas but are only seen in a vessel of the nontransgenic pancreas. (B and C, ×100.) (D and E) Gomori’s aldehyde fuchsin staining for insulin (blue cells) of an adult RIP–CD95Lhi islet (D) and a nontransgenic control islet (E). The small disorganized islets were typical of the RIP–CD95Lhi adult transgenic pancreas and contained only a few infiltrating granulocytes and macrophages. (D and E, ×200.) (F) CD95L staining (brown cells) of a RIP–CD95Lhi syngeneic (B6 to B6) islet graft after 14 days. (×100.) (G) Insulin expression (brown cells) in a nontransgenic control islet graft (B6 to B6) after 14 days. (×100.) (H and I) Insulin staining cells in allogeneic islet grafts (B6 to BALB/c) from a RIP-CD95L donor (H) or a nontransgenic donor (I) after 14 days. (×100.) (J–O) Hematoxylin/eosin staining of testis grafts recovered from BALB/c male recipients after 14 days (J–L) and after 28 days (M–O). (J–L, ×100; M–O, ×200.) (J and M) Syngeneic grafts (BALB/c). (K) Allogeneic graft (C3H). (L and O) Allogeneic grafts (C3Hgld). (N) Allogeneic graft (B6). Arrows in N and O point to regions of calcification. Sections in A–C and F were acetone-fixed frozen sections; sections in D, E, and G–O were Bouin’s solution-fixed paraffin sections.
A similar granulocytic infiltration was observed by Yagita and coworkers when they transplanted CD95L-expressing baby hamster kidney fibroblasts s.c. into nude mice (15). They hypothesized that neutrophil migration was mediated indirectly by the chemotactic cytokine interleukin 8 (IL-8). As ligation of CD95 on epithelial cells can induce secretion of IL-8 (16), we think it is likely that CD95L on the islet cells of the transgenic mice stimulated IL-8 secretion by neighboring CD95+ cells. Despite the infiltrates and fibrosis, none of the transgenic animals developed diabetes over a 30-week observation period.
CD95L Transgenic Transplants.
To test whether expression of CD95L would protect islets from rejection in allogeneic hosts, fetal pancreas grafts from C57BL/6 (B6) RIP–CD95L donors were transplanted under the kidney capsule of syngeneic (B6) or allogeneic (BALB/c) male recipients. Expression of CD95L by the syngeneic grafts in situ was confirmed by immunocytochemistry (Fig. 3F). This staining coincided with insulin staining in serial sections and thus identified the CD95L+ cells as β cells (data not shown). Syngeneic grafts from nontransgenic donors were recovered at 14 or 30 days (Table 1) and contained many islets that were free from inflammatory infiltrates (Fig. 3G). Syngeneic islets from RIP–CD95Lhi or RIP–CD95Llo donors were also recovered (Table 1) but were generally smaller, and some of them contained mild mononuclear cell infiltrates (Fig. 3F). At 30 days, two of the four RIP–CD95Lhi syngeneic grafts were rejected, presumably as a consequence of expressing CD95L.
Table 1.
Survival of RIP–CD95L fetal pancreatic grafts
| Donor | Recipient | Days | Graft survival | Appearance |
|---|---|---|---|---|
| RIP–CD95Lhi B6 | B6 | 14 | 4/4 | Transgenic islets were generally smaller and often associated with a mild infiltrate. |
| RIP–CD95Llo B6 | B6 | 14 | 3/3 | |
| Nontransgenic B6 | B6 | 14 | 2/2 | Control grafts had no infiltrate and looked healthy. |
| RIP–CD95Lhi B6 | B6 | 30 | 2/4 | Only a scar remained at two transgenic graft sites. |
| Nontransgenic B6 | B6 | 30 | 3/3 | Control grafts had no infiltrate and looked healthy. |
| RIP–CD95Lhi B6 | BALB/c | 14 | 0/5 | All graft sites were recovered but were heavily infiltrated, and no whole islets were present. |
| RIP–CD95Llo B6 | BALB/c | 14 | 0/5 | All graft sites were recovered but were heavily infiltrated, and no whole islets were present. |
| Nontransgenic B6 | BALB/c | 14 | 0/5 | All graft sites were recovered but were heavily infiltrated, and no whole islets were present. |
| RIP–CD95Lhi B6 | BALB/c | 30 | 0/3 | Only a scar remained at the graft site. |
| RIP–CD95Llo B6 | BALB/c | 30 | 0/5 | Only a scar remained at the graft site. |
| Nontransgenic B6 | BALB/c | 30 | 0/7 | Only a scar remained at the graft site. |
Nontransgenic control and RIP–CD95L transgenic grafts were recovered 14 days after transplantation into allogeneic hosts (Table 1). Both kinds of donor grafts contained heavy lymphocytic infiltrates, and only 2 of 15 had a few residual β cells and pancreatic ducts (Fig. 3 H and I). At 30 days after grafting, only a scar at the graft site remained under the kidney capsule (Table 1). Transgenic and nontransgenic B6 into BALB/c allografts were thus rejected in the same way. Neither delay in graft rejection nor increase in islet graft survival was afforded by CD95L.
Testis Transplants.
Bellgrau and coworkers reported the indefinite survival of CD95L+ B6 testis tissue grafted under the kidney capsule of allogeneic (BALB/c) recipients, whereas testis tissue from CD95L− mutant (B6gld) mice was rejected within 7 days (1). In an attempt to repeat these experiments, we grafted testis tissue from BALB/c, B6, C3H/HeJ, or C3H/HeJgld donors under the kidney capsules of male BALB/c recipients. At 14 days, syngeneic grafts had viable seminiferous tubules and were free from infiltrate (Fig. 3J). After the same period, allogeneic grafts, whether from C3H/HeJ or C3H/HeJgld donors, bore heavy lymphocytic infiltrates, and all had both damaged and viable seminiferous tubules (Fig. 3 K and L). Analogous transplantation of adrenal tissue gave similar results, although only a few residual adrenal graft cells were present in allogeneic samples (data not shown).
After 28 or 36 days, syngeneic testis grafts had viable but atrophied seminiferous tubules free from infiltrate (Fig. 3M). Allogeneic graft sites were also recovered at this time, but their gross appearance was abnormal, consisting of wide, white tubules lying under the kidney capsule. Histology showed these to be calcified seminiferous tubules associated with infiltration; no viable testis tissue remained (Fig. 3 N and O, and Table 2). C3H allografts at 28 days had the same pathology as B6 allografts (data not shown). We were therefore unable to detect any difference between the rejection of normal (CD95L+) and mutant (gld, CD95L−) allogeneic testis grafts.
Table 2.
Survival of testis grafts from CD95L+ and CD95− donors
| Donor | Recipient | Days | No. of grafts | Infiltrate* | Seminiferous tubules |
|---|---|---|---|---|---|
| BALB/c | BALB/c | 14 | 3 | All − | Viable |
| C3H | BALB/c | 14 | 7 | All +++ | Damaged and viable |
| C3Hgld | BALB/c | 14 | 8 | All +++ | Damaged and viable |
| BALB/c | BALB/c | 28 | 3 | All − | Viable, atrophied |
| B6 | BALB/c | 28 | 3 | All +++ | Necrotic, calcified |
| C3H | BALB/c | 28 | 3 | +, +, +++ | Necrotic, calcified |
| C3Hgld | BALB/c | 28 | 3 | All +++ | Necrotic, calcified |
| BALB/c | BALB/c | 36 | 3 | All − | Viable, atrophied |
| B6 | BALB/c | 36 | 3 | All ++ | Necrotic, calcified |
| C3H | BALB/c | 36 | 3 | +, +, ++ | Necrotic, calcified |
| C3Hgld | BALB/c | 36 | 3 | All + | Necrotic, calcified |
Infiltrates: +++, heavy; ++, mild; +, residual; −, none.
DISCUSSION
Historically, the testis and the eye have been considered to be immune-privileged because unmatched grafts placed into these sites are rejected more slowly than grafts placed elsewhere, such as under the kidney capsule (reviewed in ref. 3). Recent reports have shown that expression of CD95L by host cells in the eye or by syngeneic myoblasts can protect CD95L− foreign cells from immune attack (2, 17). We sought to test whether CD95L did not have to be expressed on host cells to protect foreign tissues from rejection, but would also offer protection if expressed by the graft itself as predicted by the results of Bellgrau and coworkers (1).
Our attempts to repeat the observations of Bellgrau and coworkers (1) were not successful as we found that CD95L+ or CD95L− allogeneic testis grafts developed massive lymphocytic infiltrates and lost all normal tissue architecture, with only calcified and necrotic tubules remaining by 28 days. This is in marked contrast to the observation of Bellgrau and coworkers, who reported the indefinite survival of CD95L+ allogeneic testis tissue that appeared “indistinguishable from syngeneic grafts” (1). Our findings were therefore similar to those of Statter and coworkers, who found that in both the rat and the mouse, adult allogeneic testis grafts became massively infiltrated and suffered rejection by 10 days (18, 19). We cannot account for the difference between our results and those of Bellgrau and coworkers (1).
In the pancreas of transgenic animals, CD95L provoked a granulocytic infiltrate rather than acting as an immunosuppressant. This inflammation resembled that found when baby hamster kidney cells expressing transfected CD95L were transplanted s.c. into nude mouse recipients (15). In addition, there was a remarkable absence of T or B cells in the islets, suggesting that granulocytes were specifically recruited. As ligation of CD95 on epithelial cells can induce secretion of IL-8 (16), we think it is likely that CD95L on the islet cells of the transgenic mice stimulated IL-8 secretion by neighboring CD95+ cells rather than acting on the granulocytes directly. The distribution of granulocytes in the acinar tissue of the pancreas seen in Fig. 3B could be due to a chemotactic response to a chemokine such as IL-8. As CD95L induced granulocytic infiltrates in both nude mice (15) and transgenic pancreas (Fig. 3 A and B), we do not believe T cells were involved in the induction of the granulocytic infiltrates. Backcrosses to IL-8 or IL-8 receptor-deficient mice could provide evidence for a role of this particular chemokine.
In the transgenic mice, high level expression of CD95L was detrimental to the β cells as small islets associated with fibrosis were seen in adult animals. Mice never became diabetic, however, implying either incomplete destruction or regenerative capacity of the islets. A number of mechanisms may have led to the β cell damage seen in the high expresser line, including (i) the toxic effects of overexpressed transgenes (19), (ii) the damaging effects of the granulocytic infiltrate, or (iii) the possibility that CD95 was up-regulated on transgenic islets which then killed themselves.
Islet allografts expressing CD95L were not protected from rejection. Like the nontransgenic control allografts, they were massively infiltrated with CD4+ and CD8+ T cells (data not shown). This result is not consistent with the premise of the testis grafting experiments of Bellgrau and coworkers (1), who suggested that CD95L should confer immune privilege on the tissues that express it. In fact, some CD95Lhi-expresser islet grafts failed even in syngeneic hosts (Table 1). Preliminary results in which rag-deficient mice were used as recipients of CD95L+ grafts indicate that this rejection is not mediated by T cells; nontransgenic grafts (six of six) had islets, but CD95L+ grafts (four of six) were rejected or damaged. Although granulocytes were few in these grafts, we presume it was they or some other consequence of CD95L expression that damaged the grafted transgenic tissue, just as the islets are damaged in the transgenic pancreas itself (see above). Lau and coworkers (17) found that syngeneic myoblasts expressing CD95L could protect neighboring allo-islets well enough to maintain normoglycemia in mice made diabetic with streptozotocin. Yet a similar experiment in rats showed that syngeneic, CD95L-expressing Sertoli cells could only prolong rejection of neighboring allo-islets by 9–14 days (20). This result suggests a weak immunosuppressant effect of CD95L and has only been observed when CD95L was expressed on syngeneic tissues cotransplanted with allogeneic islets.
In conclusion, we found that expression of CD95L by β cells was not sufficient to protect transgenic islet grafts from allogeneic rejection. Whether expressed by transformed (15) or nontransformed cells, CD95L acted as a proinflammatory molecule and, therefore, may contribute to rejection rather than prevent it.
Acknowledgments
We thank S. Nagata for the murine CD95L cDNA; J. Tschopp for the anti-CD95L antibody; P. Krammer for reagents; T. Mandel for help with testis grafting; and M. Ekberg, H. Thomas, L. Malcolm, T. Templeton, and S. Mihajlovic for excellent technical assistance. Thanks to W. R. Heath, S. Bennet, and J. F. A. P. Miller for their support. This work was supported by the National Health and Medical Research Council (Australia). D.L.V. and A.S. are Investigators with the Cancer Research Institute of New York, and D.L.V. is also supported by the Anti-Cancer Council of Victoria.
ABBREVIATIONS
- CD95L
CD95 ligand
- RIP
rat insulin promotor
- IL-8
interleukin 8
References
- 1.Bellgrau D, Gold D, Selawry H, Moore J, Franzusoff A, Duke R C. Nature (London) 1995;377:630–632. doi: 10.1038/377630a0. [DOI] [PubMed] [Google Scholar]
- 2.Griffith T S, Brunner T, Fletcher S M, Green D R, Ferguson T A. Science. 1995;270:1189–1192. doi: 10.1126/science.270.5239.1189. [DOI] [PubMed] [Google Scholar]
- 3.Barker C F, Billingham R E. Adv Immunol. 1977;25:1–54. [PubMed] [Google Scholar]
- 4.Nagata S, Golstein P. Science. 1995;267:1449–1456. doi: 10.1126/science.7533326. [DOI] [PubMed] [Google Scholar]
- 5.Klas C, Debatin K M, Jonker R R, Krammer P H. Int Immunol. 1993;5:625–630. doi: 10.1093/intimm/5.6.625. [DOI] [PubMed] [Google Scholar]
- 6.Vaux D L. Nature (London) 1995;377:576–577. doi: 10.1038/377576a0. [DOI] [PubMed] [Google Scholar]
- 7.Hanahan D. Nature (London) 1985;315:115–122. doi: 10.1038/315115a0. [DOI] [PubMed] [Google Scholar]
- 8.Takahashi T, Tanaka M, Brannan C I, Jenkins N A, Copeland N G, Suda T, Nagata S. Cell. 1994;76:969–976. doi: 10.1016/0092-8674(94)90375-1. [DOI] [PubMed] [Google Scholar]
- 9.Lake S P, Anderson J, Chamberlain J, Gardner S J, Bell P R, James R F. Transplantation. 1987;43:805–808. [PubMed] [Google Scholar]
- 10.Dhein J, Walczak H, Baumler C, Debatin K M, Krammer P H. Nature (London) 1995;373:438–441. doi: 10.1038/373438a0. [DOI] [PubMed] [Google Scholar]
- 11.Trauth B C, Klas C, Peters A M, Matzku S, Moller P, Falk W, Debatin K M, Krammer P H. Science. 1989;245:301–305. doi: 10.1126/science.2787530. [DOI] [PubMed] [Google Scholar]
- 12.Georgiou H M, Mandel T E. Transplant Proc. 1986;18:319–321. [PubMed] [Google Scholar]
- 13.Allison J, Campbell I L, Morahan G, Mandel T E, Harrison L C, Miller J F. Nature (London) 1988;333:529–533. doi: 10.1038/333529a0. [DOI] [PubMed] [Google Scholar]
- 14.French L E, Hahne M, Viard I, Radlgruber G, Zanone R, Becker K, Muller C, Tschopp J. J Cell Biol. 1996;133:335–343. doi: 10.1083/jcb.133.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yagita H, Seino K, Kayagaki N, Okumura K. Nature (London) 1996;379:682. doi: 10.1038/379682a0. [DOI] [PubMed] [Google Scholar]
- 16.Abreu M M, Vidrich A, Lynch D H, Targan S R. J Immunol. 1995;155:4147–4154. [PubMed] [Google Scholar]
- 17.Lau H T, Yu M, Fontana A, Stoeckert C J. Science. 1996;273:109–112. doi: 10.1126/science.273.5271.109. [DOI] [PubMed] [Google Scholar]
- 18.Statter M B, Foglia R P, Parks D E, Donahoe P K. J Urol. 1988;139:204–210. doi: 10.1016/s0022-5347(17)42354-8. [DOI] [PubMed] [Google Scholar]
- 19.Statter M B, Fahrner K J, Barksdale E J, Parks D E, Flavell R A, Donahoe P K. Transplantation. 1989;47:651–660. doi: 10.1097/00007890-198904000-00017. [DOI] [PubMed] [Google Scholar]
- 20.Selawry H P, Cameron D F. Cell Transplant. 1993;2:123–129. doi: 10.1177/096368979300200206. [DOI] [PubMed] [Google Scholar]