Abstract
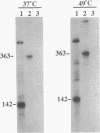
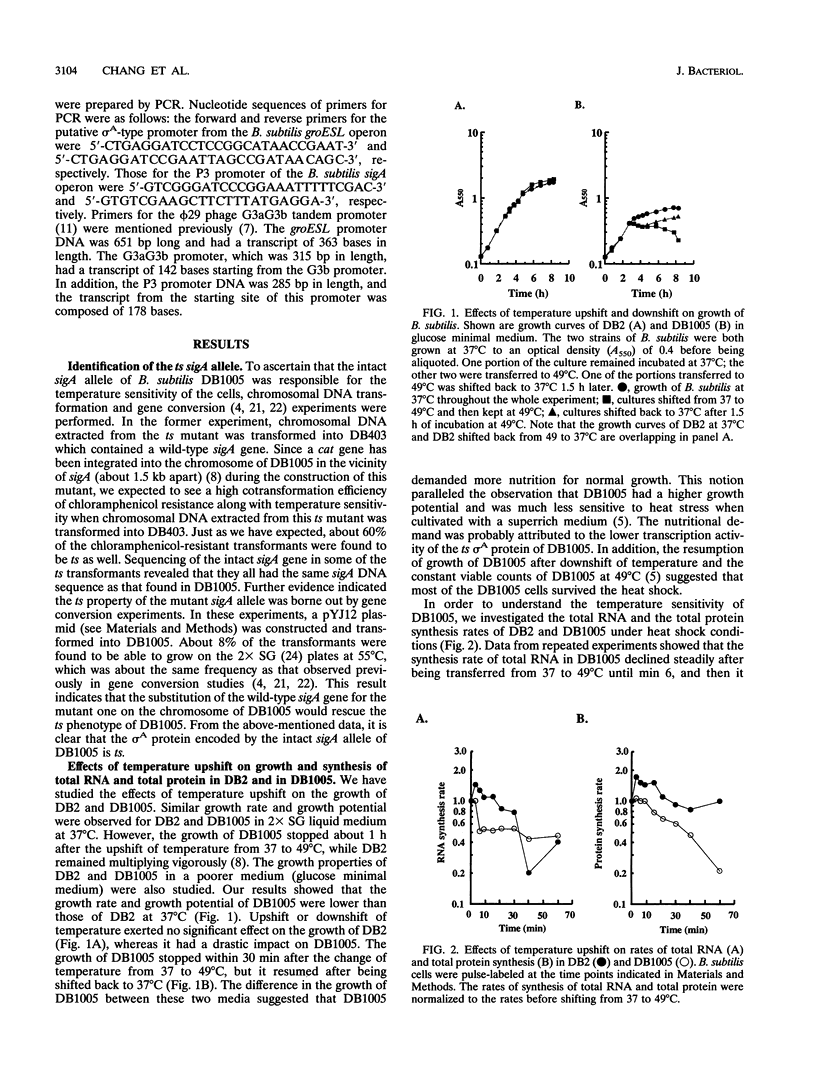
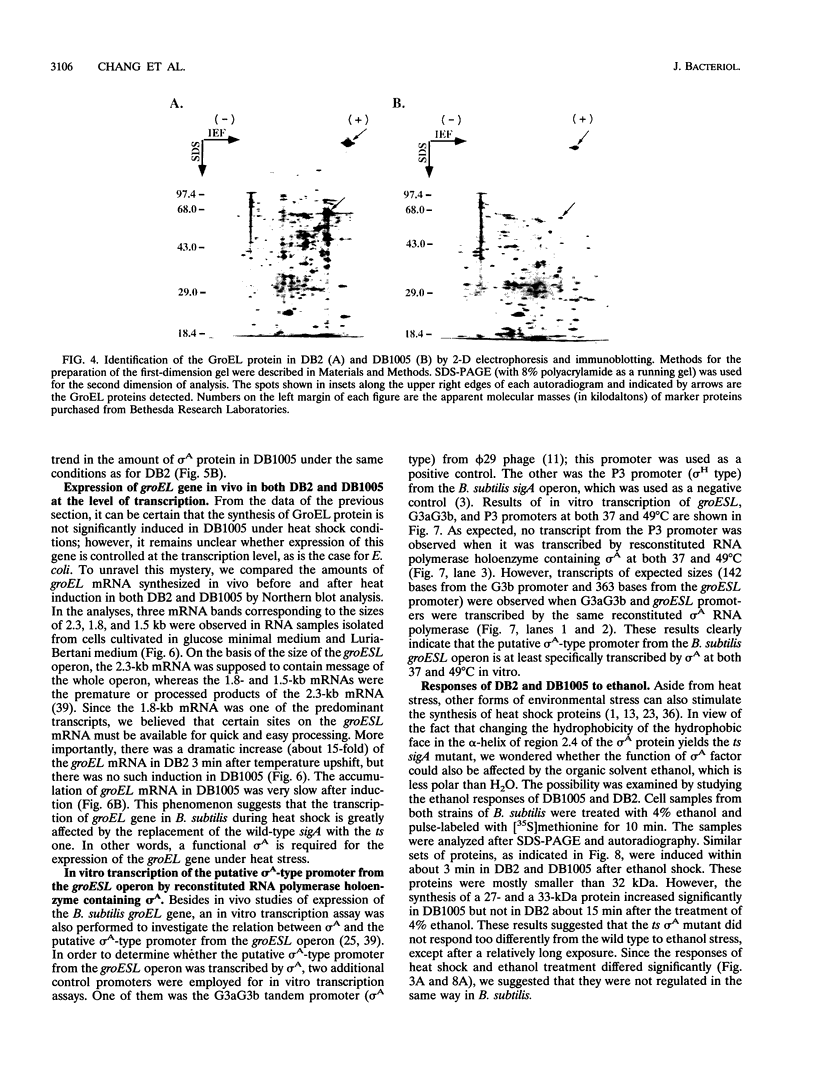
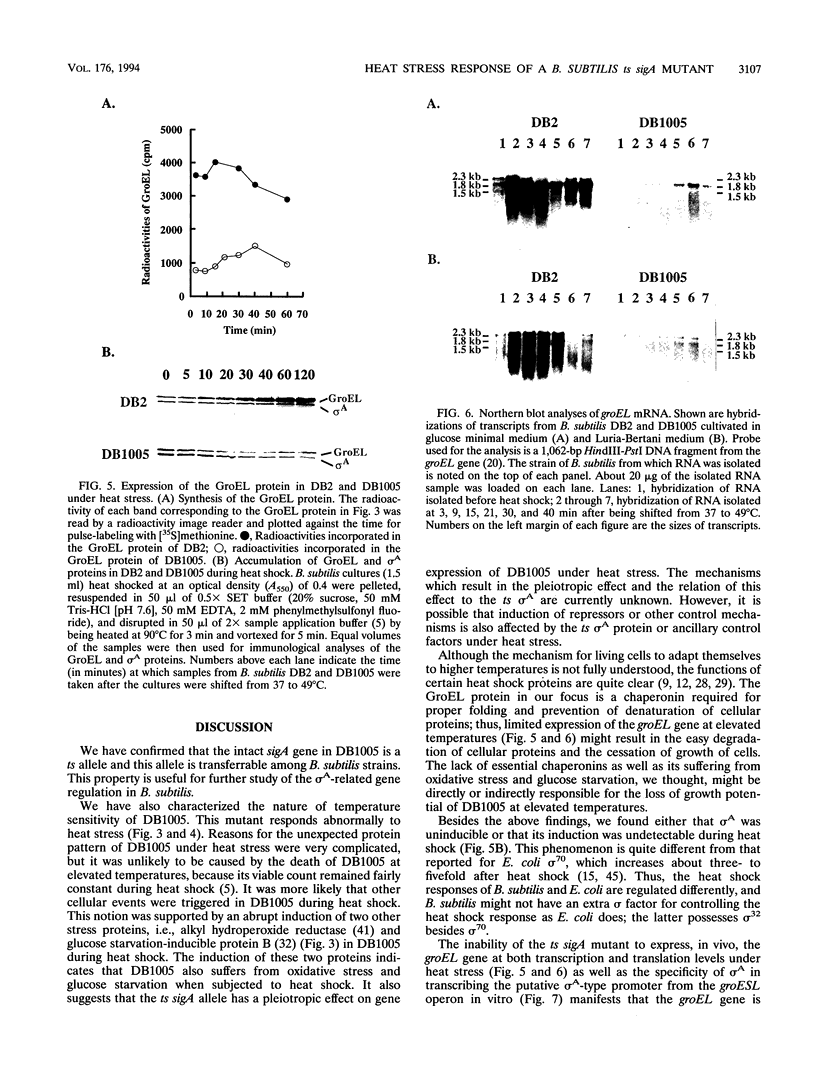
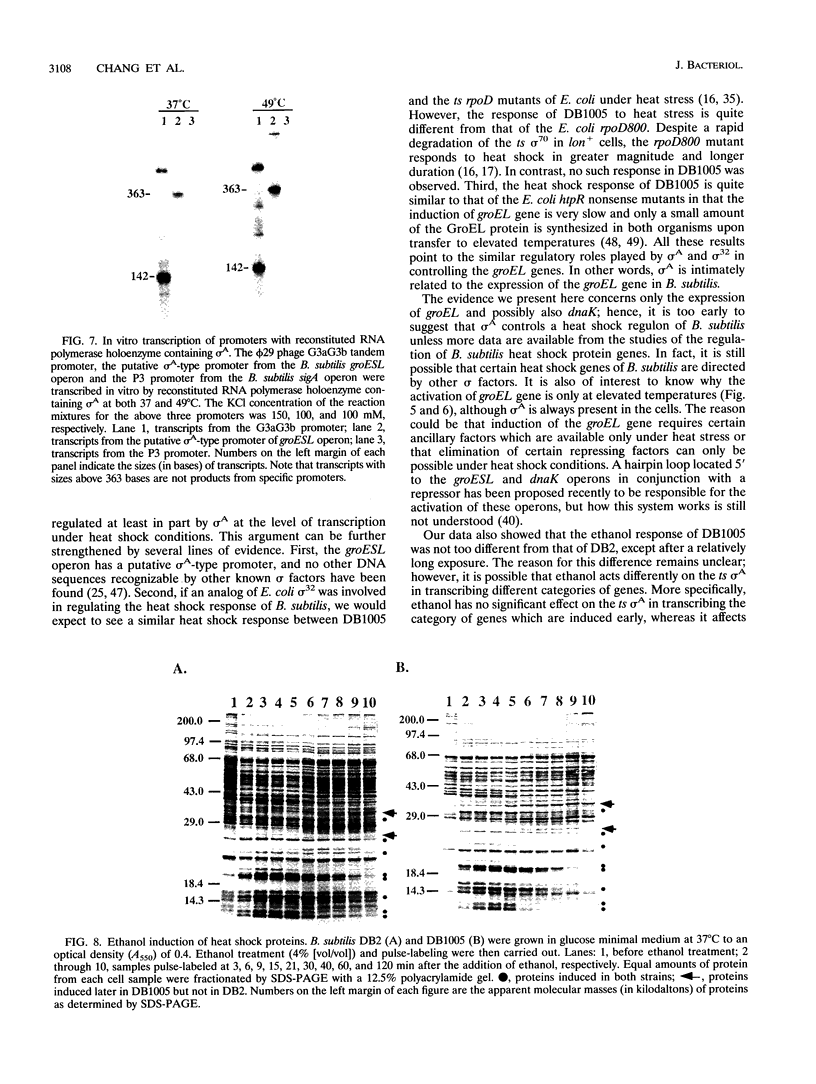
The mutant sigA allele of Bacillus subtilis DB1005 was confirmed to be temperature sensitive (ts) and transferable among strains of B. subtilis by chromosomal transformation and gene conversion. This ts sigA allele had a pleiotropic effect on gene expression of DB1005. The induction of certain heat shock proteins in DB1005 was markedly less significant than that observed in the wild-type strain (DB2) under heat stress. In contrast, some proteins required for coping with oxidative stress and glucose starvation were induced abruptly in DB1005 but not in DB2. Heat induction of the groEL gene in vivo at both transcription and translation levels was much lower in DB1005 than in DB2. Besides, the putative sigma A-type promoter from the groESL operon of B. subtilis was able to be transcribed by the reconstituted sigma A RNA polymerase in vitro at both 37 and 49 degrees C. These results strongly suggest that the expression of the groEL gene of B. subtilis under heat stress is regulated at least in part by sigma A at the level of transcription. Our results also showed that DB1005 did not respond too differently from the wild type to ethanol stress, except after a relatively long exposure.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnosti D. N., Singer V. L., Chamberlin M. J. Characterization of heat shock in Bacillus subtilis. J Bacteriol. 1986 Dec;168(3):1243–1249. doi: 10.1128/jb.168.3.1243-1249.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briat J. F., Gilman M. Z., Chamberlin M. J. Bacillus subtilis sigma 28 and Escherichia coli sigma 32 (htpR) are minor sigma factors that display an overlapping promoter specificity. J Biol Chem. 1985 Feb 25;260(4):2038–2041. [PubMed] [Google Scholar]
- Carter H. L., 3rd, Wang L. F., Doi R. H., Moran C. P., Jr rpoD operon promoter used by sigma H-RNA polymerase in Bacillus subtilis. J Bacteriol. 1988 Apr;170(4):1617–1621. doi: 10.1128/jb.170.4.1617-1621.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chak K. F., de Lencastre H., Liu H. M., Piggot P. J. Facile in vivo transfer of mutations between the Bacillus subtilis chromosome and a plasmid harbouring homologous DNA. J Gen Microbiol. 1982 Nov;128(11):2813–2816. doi: 10.1099/00221287-128-11-2813. [DOI] [PubMed] [Google Scholar]
- Chang B. Y., Doi R. H. Conformational properties of Bacillus subtilis RNA polymerase sigma A factor during transcription initiation. Biochem J. 1993 Aug 15;294(Pt 1):43–47. doi: 10.1042/bj2940043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang B. Y., Doi R. H. Effects of amino acid substitutions in the promoter -10 binding region of the sigma A factor on growth of Bacillus subtilis. J Bacteriol. 1993 Apr;175(8):2470–2474. doi: 10.1128/jb.175.8.2470-2474.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang B. Y., Doi R. H. Overproduction, purification, and characterization of Bacillus subtilis RNA polymerase sigma A factor. J Bacteriol. 1990 Jun;172(6):3257–3263. doi: 10.1128/jb.172.6.3257-3263.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung C. H., Goldberg A. L. The product of the lon (capR) gene in Escherichia coli is the ATP-dependent protease, protease La. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4931–4935. doi: 10.1073/pnas.78.8.4931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davison B. L., Leighton T., Rabinowitz J. C. Purification of Bacillus subtilis RNA polymerase with heparin-agarose. In vitro transcription of phi 29 DNA. J Biol Chem. 1979 Sep 25;254(18):9220–9226. [PubMed] [Google Scholar]
- Davison B. L., Murray C. L., Rabinowitz J. C. Specificity of promoter site utilization in vitro by bacterial RNA polymerases on Bacillus phage phi 29 DNA. Transcription mapping with exonuclease III. J Biol Chem. 1980 Sep 25;255(18):8819–8830. [PubMed] [Google Scholar]
- Deshaies R. J., Koch B. D., Werner-Washburne M., Craig E. A., Schekman R. A subfamily of stress proteins facilitates translocation of secretory and mitochondrial precursor polypeptides. Nature. 1988 Apr 28;332(6167):800–805. doi: 10.1038/332800a0. [DOI] [PubMed] [Google Scholar]
- Drahos D. J., Hendrix R. W. Effect of bacteriophage lambda infection on synthesis of groE protein and other Escherichia coli proteins. J Bacteriol. 1982 Mar;149(3):1050–1063. doi: 10.1128/jb.149.3.1050-1063.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda R., Doi R. H. Two polypeptides associated with the ribonucleic acid polymerase core of Bacillus subtilis during sporulation. J Bacteriol. 1977 Jan;129(1):422–432. doi: 10.1128/jb.129.1.422-432.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross C. A., Grossman A. D., Liebke H., Walter W., Burgess R. R. Effects of the mutant sigma allele rpoD800 on the synthesis of specific macromolecular components of the Escherichia coli K12 cell. J Mol Biol. 1984 Jan 25;172(3):283–300. doi: 10.1016/s0022-2836(84)80027-3. [DOI] [PubMed] [Google Scholar]
- Grossman A. D., Burgess R. R., Walter W., Gross C. A. Mutations in the Ion gene of E. coli K12 phenotypically suppress a mutation in the sigma subunit of RNA polymerase. Cell. 1983 Jan;32(1):151–159. doi: 10.1016/0092-8674(83)90505-6. [DOI] [PubMed] [Google Scholar]
- Grossman A. D., Erickson J. W., Gross C. A. The htpR gene product of E. coli is a sigma factor for heat-shock promoters. Cell. 1984 Sep;38(2):383–390. doi: 10.1016/0092-8674(84)90493-8. [DOI] [PubMed] [Google Scholar]
- Halling S. M., Sanchez-Anzaldo F. J., Fukuda R., Doi R. H., Meares C. F. Zinc is associated with the beta subunit of DNA-dependent RNA polymerase of Bacillus subtilis. Biochemistry. 1977 Jun 28;16(13):2880–2884. doi: 10.1021/bi00632a012. [DOI] [PubMed] [Google Scholar]
- Helmann J. D., Márquez L. M., Chamberlin M. J. Cloning, sequencing, and disruption of the Bacillus subtilis sigma 28 gene. J Bacteriol. 1988 Apr;170(4):1568–1574. doi: 10.1128/jb.170.4.1568-1574.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura F., Wang L. F., Doi R. H. Catabolite-resistant sporulation (crsA) mutations in the Bacillus subtilis RNA polymerase sigma 43 gene (rpoD) can suppress and be suppressed by mutations in spo0 genes. Proc Natl Acad Sci U S A. 1985 Dec;82(23):8124–8128. doi: 10.1073/pnas.82.23.8124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger J. H., Walker G. C. groEL and dnaK genes of Escherichia coli are induced by UV irradiation and nalidixic acid in an htpR+-dependent fashion. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1499–1503. doi: 10.1073/pnas.81.5.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leighton T. J., Doi R. H. The stability of messenger ribonucleic acid during sporulation in Bacillus subtilis. J Biol Chem. 1971 May 25;246(10):3189–3195. [PubMed] [Google Scholar]
- Li M., Wong S. L. Cloning and characterization of the groESL operon from Bacillus subtilis. J Bacteriol. 1992 Jun;174(12):3981–3992. doi: 10.1128/jb.174.12.3981-3992.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist S., Craig E. A. The heat-shock proteins. Annu Rev Genet. 1988;22:631–677. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- Lindquist S. The heat-shock response. Annu Rev Biochem. 1986;55:1151–1191. doi: 10.1146/annurev.bi.55.070186.005443. [DOI] [PubMed] [Google Scholar]
- Martin J., Horwich A. L., Hartl F. U. Prevention of protein denaturation under heat stress by the chaperonin Hsp60. Science. 1992 Nov 6;258(5084):995–998. doi: 10.1126/science.1359644. [DOI] [PubMed] [Google Scholar]
- Martin J., Langer T., Boteva R., Schramel A., Horwich A. L., Hartl F. U. Chaperonin-mediated protein folding at the surface of groEL through a 'molten globule'-like intermediate. Nature. 1991 Jul 4;352(6330):36–42. doi: 10.1038/352036a0. [DOI] [PubMed] [Google Scholar]
- Mirel D. B., Chamberlin M. J. The Bacillus subtilis flagellin gene (hag) is transcribed by the sigma 28 form of RNA polymerase. J Bacteriol. 1989 Jun;171(6):3095–3101. doi: 10.1128/jb.171.6.3095-3101.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller J. P., Bukusoglu G., Sonenshein A. L. Transcriptional regulation of Bacillus subtilis glucose starvation-inducible genes: control of gsiA by the ComP-ComA signal transduction system. J Bacteriol. 1992 Jul;174(13):4361–4373. doi: 10.1128/jb.174.13.4361-4373.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neidhardt F. C., VanBogelen R. A., Vaughn V. The genetics and regulation of heat-shock proteins. Annu Rev Genet. 1984;18:295–329. doi: 10.1146/annurev.ge.18.120184.001455. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Osawa T., Yura T. Effects of reduced amount of RNA polymerase sigma factor on gene expression and growth of Escherichia coli: studies of the rpoD450 (amber) mutation. Mol Gen Genet. 1981;184(2):166–173. doi: 10.1007/BF00272900. [DOI] [PubMed] [Google Scholar]
- Plesset J., Palm C., McLaughlin C. S. Induction of heat shock proteins and thermotolerance by ethanol in Saccharomyces cerevisiae. Biochem Biophys Res Commun. 1982 Oct 15;108(3):1340–1345. doi: 10.1016/0006-291x(82)92147-7. [DOI] [PubMed] [Google Scholar]
- Rosenberg A. H., Lade B. N., Chui D. S., Lin S. W., Dunn J. J., Studier F. W. Vectors for selective expression of cloned DNAs by T7 RNA polymerase. Gene. 1987;56(1):125–135. doi: 10.1016/0378-1119(87)90165-x. [DOI] [PubMed] [Google Scholar]
- Schmidt A., Schiesswohl M., Völker U., Hecker M., Schumann W. Cloning, sequencing, mapping, and transcriptional analysis of the groESL operon from Bacillus subtilis. J Bacteriol. 1992 Jun;174(12):3993–3999. doi: 10.1128/jb.174.12.3993-3999.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schön U., Schumann W. Molecular cloning, sequencing, and transcriptional analysis of the groESL operon from Bacillus stearothermophilus. J Bacteriol. 1993 Apr;175(8):2465–2469. doi: 10.1128/jb.175.8.2465-2469.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smillie D. A., Hayward R. S., Suzuki T., Fujita N., Ishihama A. Locations of genes encoding alkyl hydroperoxide reductase on the physical map of the Escherichia coli K-12 genome. J Bacteriol. 1992 Jun;174(11):3826–3827. doi: 10.1128/jb.174.11.3826-3827.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spizizen J. TRANSFORMATION OF BIOCHEMICALLY DEFICIENT STRAINS OF BACILLUS SUBTILIS BY DEOXYRIBONUCLEATE. Proc Natl Acad Sci U S A. 1958 Oct 15;44(10):1072–1078. doi: 10.1073/pnas.44.10.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straus D. B., Walter W. A., Gross C. A. The heat shock response of E. coli is regulated by changes in the concentration of sigma 32. Nature. 1987 Sep 24;329(6137):348–351. doi: 10.1038/329348a0. [DOI] [PubMed] [Google Scholar]
- Streips U. N., Polio F. W. Heat shock proteins in bacilli. J Bacteriol. 1985 Apr;162(1):434–437. doi: 10.1128/jb.162.1.434-437.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor W. E., Straus D. B., Grossman A. D., Burton Z. F., Gross C. A., Burgess R. R. Transcription from a heat-inducible promoter causes heat shock regulation of the sigma subunit of E. coli RNA polymerase. Cell. 1984 Sep;38(2):371–381. doi: 10.1016/0092-8674(84)90492-6. [DOI] [PubMed] [Google Scholar]
- Wetzstein M., Völker U., Dedio J., Löbau S., Zuber U., Schiesswohl M., Herget C., Hecker M., Schumann W. Cloning, sequencing, and molecular analysis of the dnaK locus from Bacillus subtilis. J Bacteriol. 1992 May;174(10):3300–3310. doi: 10.1128/jb.174.10.3300-3310.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yura T., Tobe T., Ito K., Osawa T. Heat shock regulatory gene (htpR) of Escherichia coli is required for growth at high temperature but is dispensable at low temperature. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6803–6807. doi: 10.1073/pnas.81.21.6803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y. N., Gross C. A. How a mutation in the gene encoding sigma 70 suppresses the defective heat shock response caused by a mutation in the gene encoding sigma 32. J Bacteriol. 1992 Nov;174(22):7128–7137. doi: 10.1128/jb.174.22.7128-7137.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]