Abstract
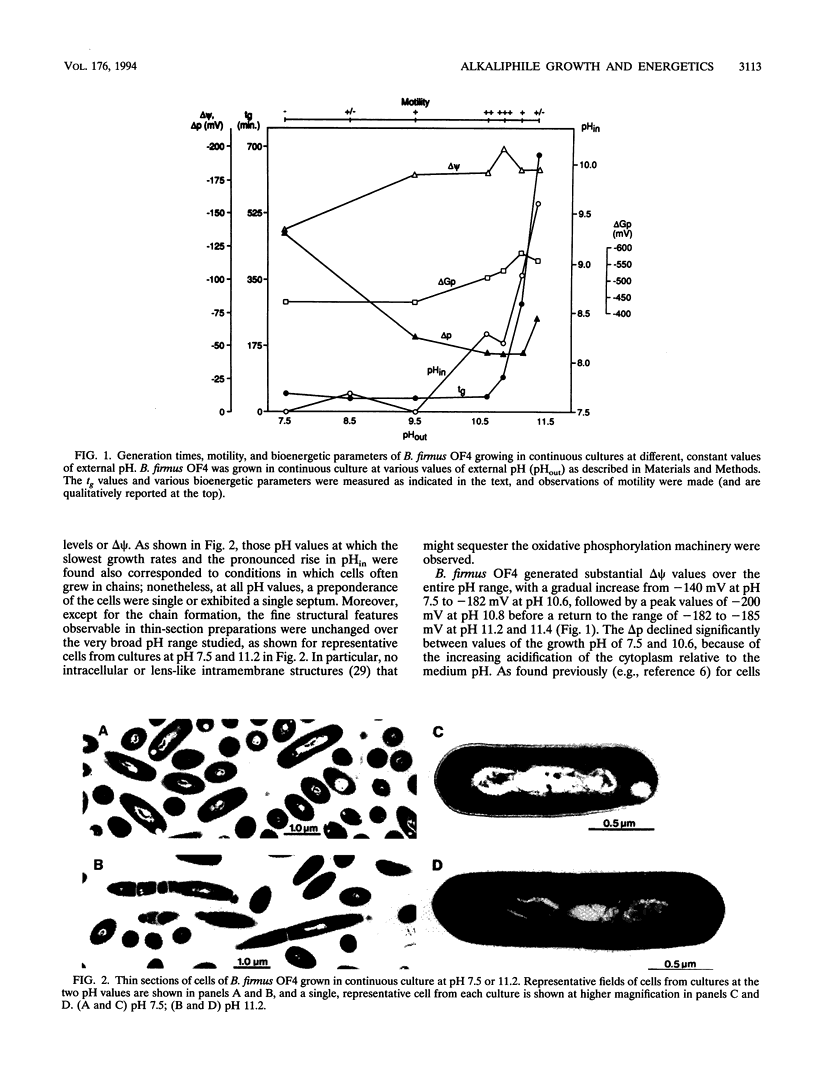
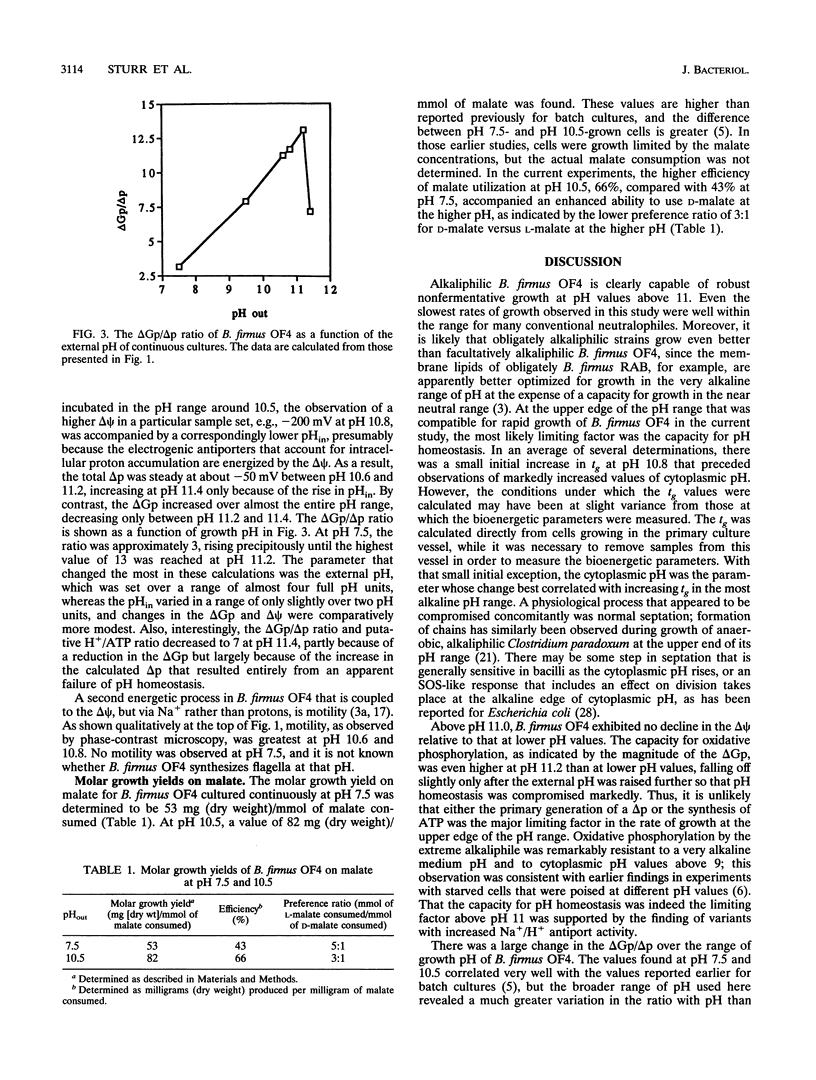
The effect of external pH on growth of alkaliphilic Bacillus firmus OF4 was studied in steady-state, pH-controlled cultures at various pH values. Generation times of 54 and 38 min were observed at external pH values of 7.5 and 10.6, respectively. At more alkaline pH values, generation times increased, reaching 690 min at pH 11.4; this was approximately the upper limit of pH for growth with doubling times below 12 h. Decreasing growth rates above pH 11 correlated with an apparent decrease in the ability to tightly regulate cytoplasmic pH and with the appearance of chains of cells. Whereas the cytoplasmic pH was maintained at pH 8.3 or below up to external pH values of 10.8, there was an increase up to pH 8.9 and 9.6 as the growth pH was increased to 11.2 and 11.4, respectively. Both the transmembrane electrical potential and the phosphorylation potential (delta Gp) generally increased over the total pH range, except for a modest fall-off in the delta Gp at pH 11.4. The capacity for pH homeostasis rather than that for oxidative phosphorylation first appeared to become limiting for growth at the high edge of the pH range. No cytoplasmic or membrane-associated organelles were observed at any growth pH, confirming earlier conclusions that structural sequestration of oxidative phosphorylation was not used to resolve the discordance between the total electrochemical proton gradient (delta p) and the delta Gp as the external pH is raised. Were a strictly bulk chemiosmotic coupling mechanism to account for oxidative phosphorylation over the entire range, the deltaGp/deltap ration (which would equal the H+/ATP ratio) would rise from about 3 at pH 7.5 to 13 at pH 11.2, dropping to 7 at pH 11.4 only because of the rise in cytoplasmic pH relative to other parameters. Moreover, the molar growth yields on malate were higher at pH 10.5 than at pH 7.5, indicating greater rather than lesser efficiency in the use of substrate at the more alkaline pH.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Clejan S., Krulwich T. A., Mondrus K. R., Seto-Young D. Membrane lipid composition of obligately and facultatively alkalophilic strains of Bacillus spp. J Bacteriol. 1986 Oct;168(1):334–340. doi: 10.1128/jb.168.1.334-340.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunkley E. A., Jr, Guffanti A. A., Clejan S., Krulwich T. A. Facultative alkaliphiles lack fatty acid desaturase activity and lose the ability to grow at near-neutral pH when supplemented with an unsaturated fatty acid. J Bacteriol. 1991 Feb;173(3):1331–1334. doi: 10.1128/jb.173.3.1331-1334.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guffanti A. A., Finkelthal O., Hicks D. B., Falk L., Sidhu A., Garro A., Krulwich T. A. Isolation and characterization of new facultatively alkalophilic strains of Bacillus species. J Bacteriol. 1986 Sep;167(3):766–773. doi: 10.1128/jb.167.3.766-773.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guffanti A. A., Hicks D. B. Molar growth yields and bioenergetic parameters of extremely alkaliphilic Bacillus species in batch cultures, and growth in a chemostat at pH 10.5. J Gen Microbiol. 1991 Oct;137(10):2375–2379. doi: 10.1099/00221287-137-10-2375. [DOI] [PubMed] [Google Scholar]
- Guffanti A. A., Krulwich T. A. Features of apparent nonchemiosmotic energization of oxidative phosphorylation by alkaliphilic Bacillus firmus OF4. J Biol Chem. 1992 May 15;267(14):9580–9588. [PubMed] [Google Scholar]
- Hernandez E., Johnson M. J. Energy supply and cell yield in aerobically growth microorganisms. J Bacteriol. 1967 Oct;94(4):996–1001. doi: 10.1128/jb.94.4.996-1001.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks D. B., Krulwich T. A. Purification and reconstitution of the F1F0-ATP synthase from alkaliphilic Bacillus firmus OF4. Evidence that the enzyme translocates H+ but not Na+. J Biol Chem. 1990 Nov 25;265(33):20547–20554. [PubMed] [Google Scholar]
- Hoffmann A., Dimroth P. The ATPase of Bacillus alcalophilus. Reconstitution of energy-transducing functions. Eur J Biochem. 1991 Mar 14;196(2):493–497. doi: 10.1111/j.1432-1033.1991.tb15841.x. [DOI] [PubMed] [Google Scholar]
- Hoffmann A., Dimroth P. The electrochemical proton potential of Bacillus alcalophilus. Eur J Biochem. 1991 Oct 15;201(2):467–473. doi: 10.1111/j.1432-1033.1991.tb16304.x. [DOI] [PubMed] [Google Scholar]
- Ivey D. M., Krulwich T. A. Two unrelated alkaliphilic Bacillus species possess identical deviations in sequence from those of other prokaryotes in regions of F0 proposed to be involved in proton translocation through the ATP synthase. Res Microbiol. 1992 Jun;143(5):467–470. doi: 10.1016/0923-2508(92)90092-3. [DOI] [PubMed] [Google Scholar]
- Kates M., Syz J. Y., Gosser D., Haines T. H. pH-dissociation characteristics of cardiolipin and its 2'-deoxy analogue. Lipids. 1993 Oct;28(10):877–882. doi: 10.1007/BF02537494. [DOI] [PubMed] [Google Scholar]
- Khan S., Ivey D. M., Krulwich T. A. Membrane ultrastructure of alkaliphilic Bacillus species studied by rapid-freeze electron microscopy. J Bacteriol. 1992 Aug;174(15):5123–5126. doi: 10.1128/jb.174.15.5123-5126.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitada M., Guffanti A. A., Krulwich T. A. Bioenergetic properties and viability of alkalophilic Bacillus firmus RAB as a function of pH and Na+ contents of the incubation medium. J Bacteriol. 1982 Dec;152(3):1096–1104. doi: 10.1128/jb.152.3.1096-1104.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krenn B. E., Van Walraven H. S., Scholts M. J., Kraayenhof R. Modulation of the proton-translocation stoichiometry of H(+)-ATP synthases in two phototrophic prokaryotes by external pH. Biochem J. 1993 Sep 15;294(Pt 3):705–709. doi: 10.1042/bj2940705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krulwich T. A., Guffanti A. A. Proton-coupled bioenergetic processes in extremely alkaliphilic bacteria. J Bioenerg Biomembr. 1992 Dec;24(6):587–599. doi: 10.1007/BF00762351. [DOI] [PubMed] [Google Scholar]
- LeBel D., Poirier G. G., Beaudoin A. R. A convenient method for the ATPase assay. Anal Biochem. 1978 Mar;85(1):86–89. doi: 10.1016/0003-2697(78)90277-4. [DOI] [PubMed] [Google Scholar]
- Payne W. J. Energy yields and growth of heterotrophs. Annu Rev Microbiol. 1970;24:17–52. doi: 10.1146/annurev.mi.24.100170.000313. [DOI] [PubMed] [Google Scholar]
- Quirk P. G., Guffanti A. A., Plass R. J., Clejan S., Krulwich T. A. Protonophore-resistance and cytochrome expression in mutant strains of the facultative alkaliphile Bacillus firmus OF4. Biochim Biophys Acta. 1991 Jun 17;1058(2):131–140. doi: 10.1016/s0005-2728(05)80229-4. [DOI] [PubMed] [Google Scholar]
- Quirk P. G., Hicks D. B., Krulwich T. A. Cloning of the cta operon from alkaliphilic Bacillus firmus OF4 and characterization of the pH-regulated cytochrome caa3 oxidase it encodes. J Biol Chem. 1993 Jan 5;268(1):678–685. [PubMed] [Google Scholar]
- Rohde M., Mayer F., Hicks D. B., Krulwich T. A. Immunoelectron microscopic localization of the F1F0 ATPase (ATP synthase) on the cytoplasmic membrane of alkalophilic Bacillus firmus RAB. Biochim Biophys Acta. 1989 Oct 16;985(2):233–235. doi: 10.1016/0005-2736(89)90369-6. [DOI] [PubMed] [Google Scholar]
- Rosing J., Slater E. C. The value of G degrees for the hydrolysis of ATP. Biochim Biophys Acta. 1972 May 25;267(2):275–290. doi: 10.1016/0005-2728(72)90116-8. [DOI] [PubMed] [Google Scholar]
- Schuldiner S., Agmon V., Brandsma J., Cohen A., Friedman E., Padan E. Induction of SOS functions by alkaline intracellular pH in Escherichia coli. J Bacteriol. 1986 Nov;168(2):936–939. doi: 10.1128/jb.168.2.936-939.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skulachev V. P. Chemiosmotic systems in bioenergetics: H(+)-cycles and Na(+)-cycles. Biosci Rep. 1991 Dec;11(6):387–444. doi: 10.1007/BF01130214. [DOI] [PubMed] [Google Scholar]
- Tocanne J. F., Teissié J. Ionization of phospholipids and phospholipid-supported interfacial lateral diffusion of protons in membrane model systems. Biochim Biophys Acta. 1990 Feb 28;1031(1):111–142. doi: 10.1016/0304-4157(90)90005-w. [DOI] [PubMed] [Google Scholar]
- Zilberstein D., Agmon V., Schuldiner S., Padan E. The sodium/proton antiporter is part of the pH homeostasis mechanism in Escherichia coli. J Biol Chem. 1982 Apr 10;257(7):3687–3691. [PubMed] [Google Scholar]




