Abstract
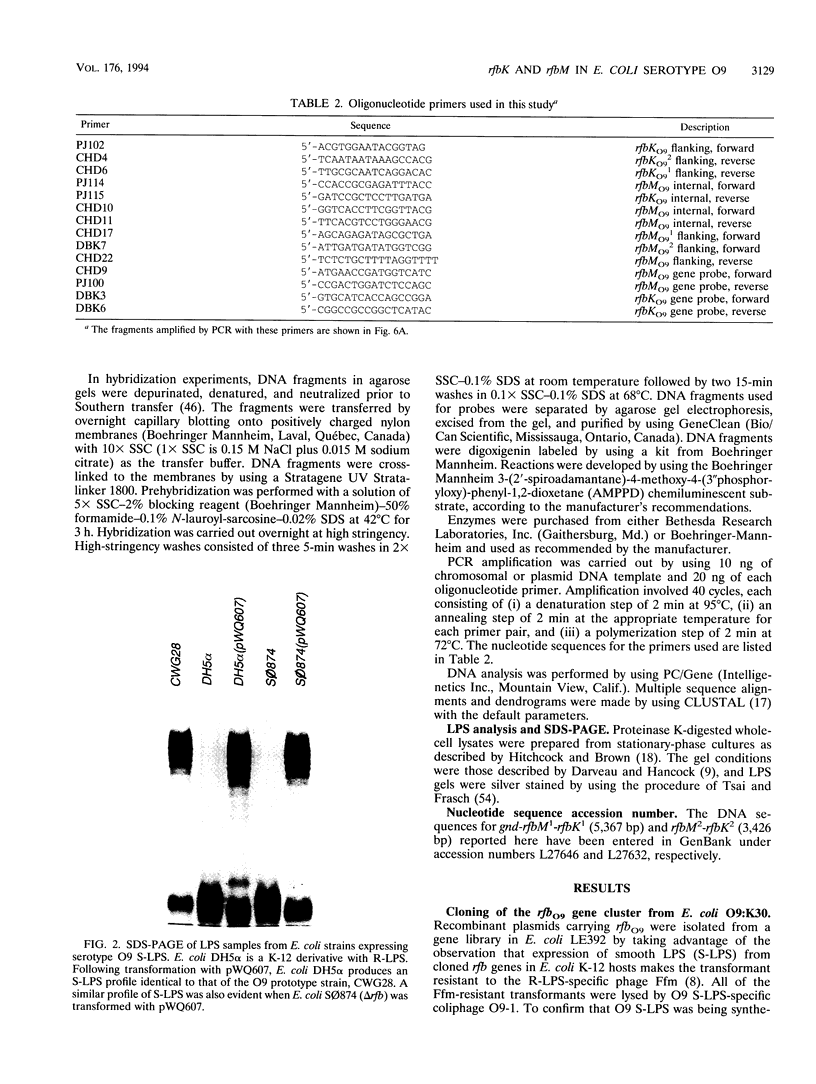
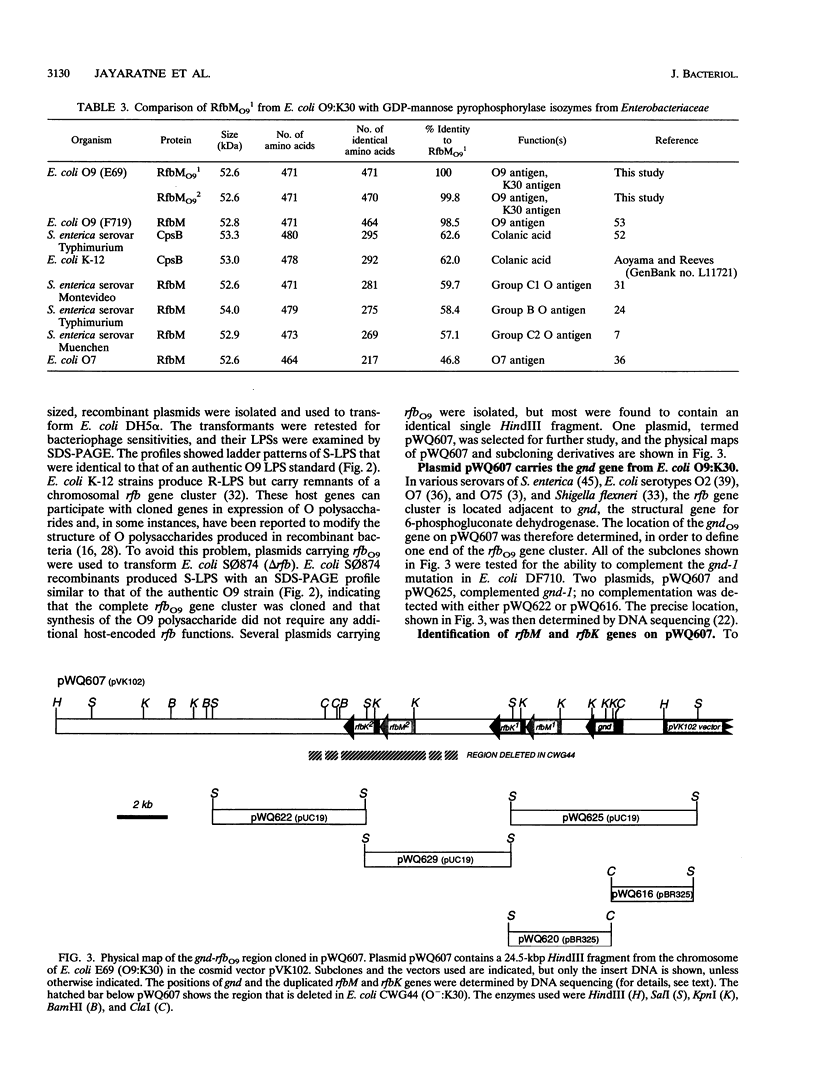
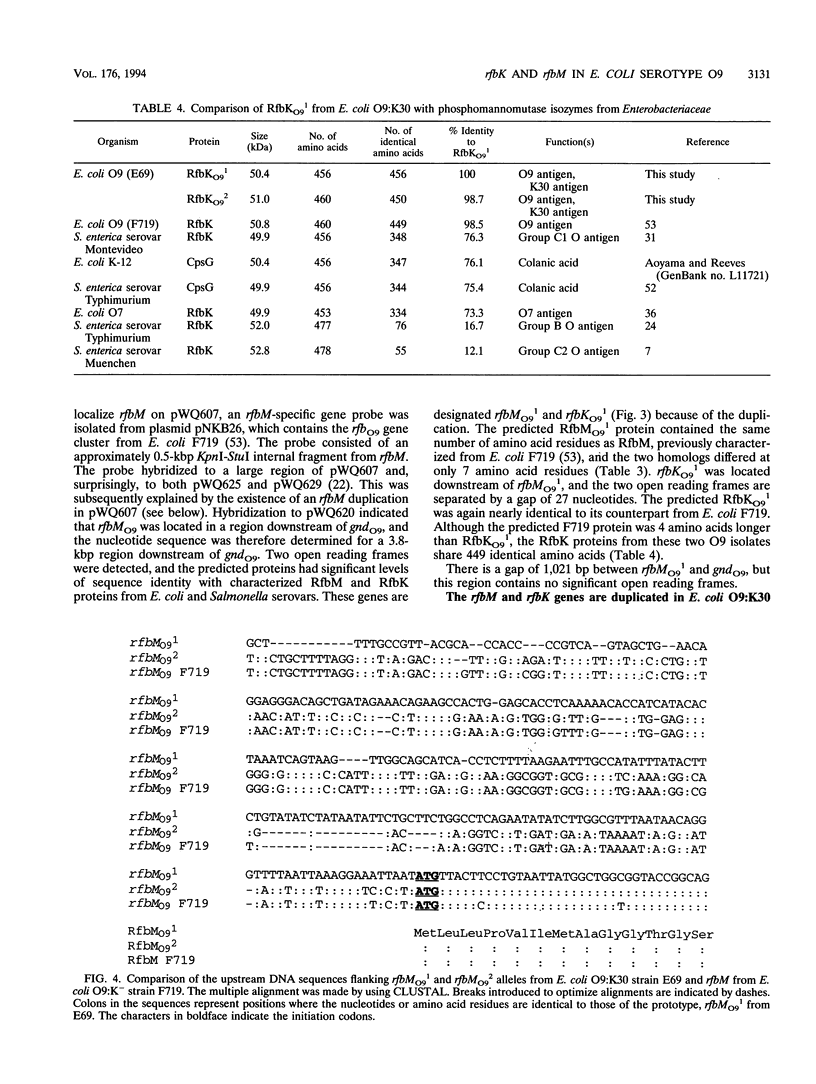
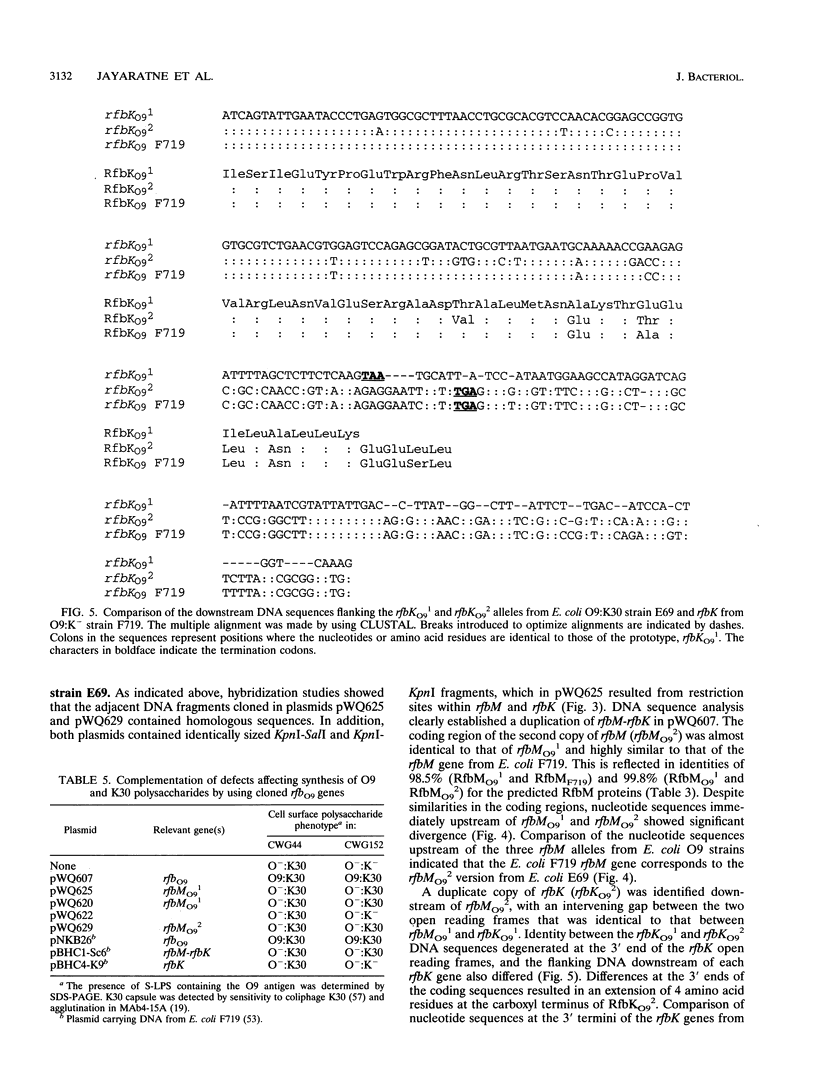
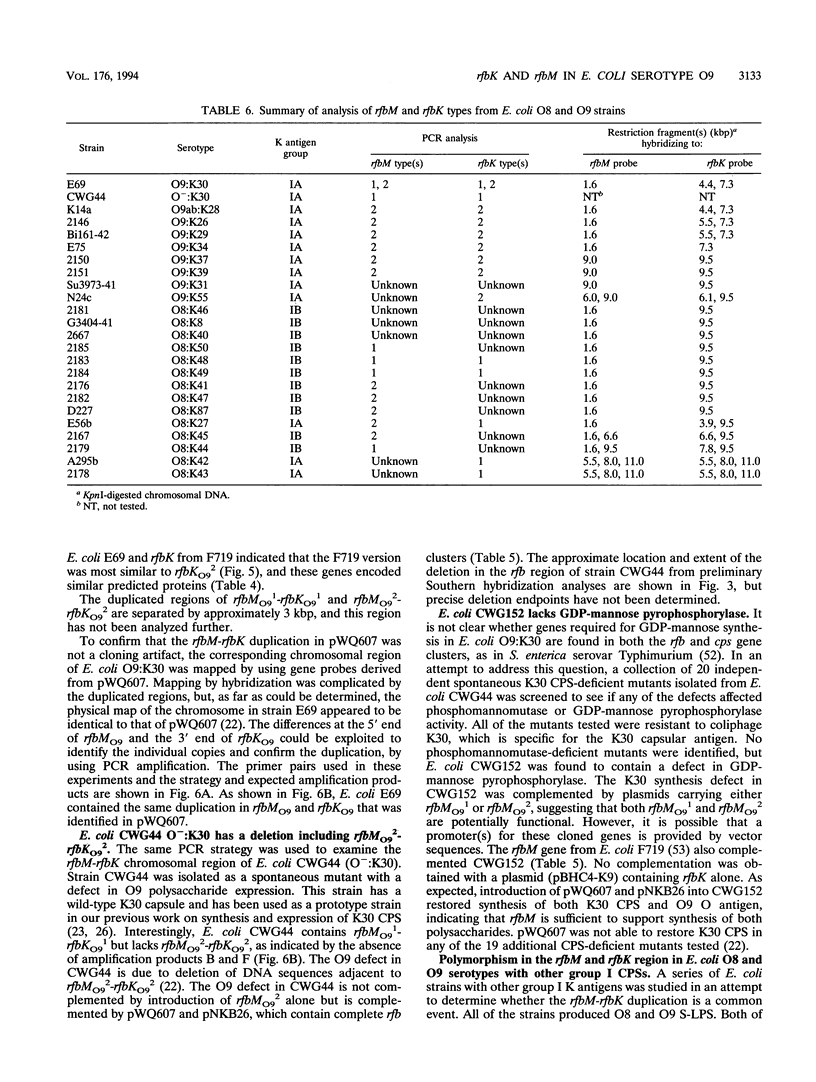
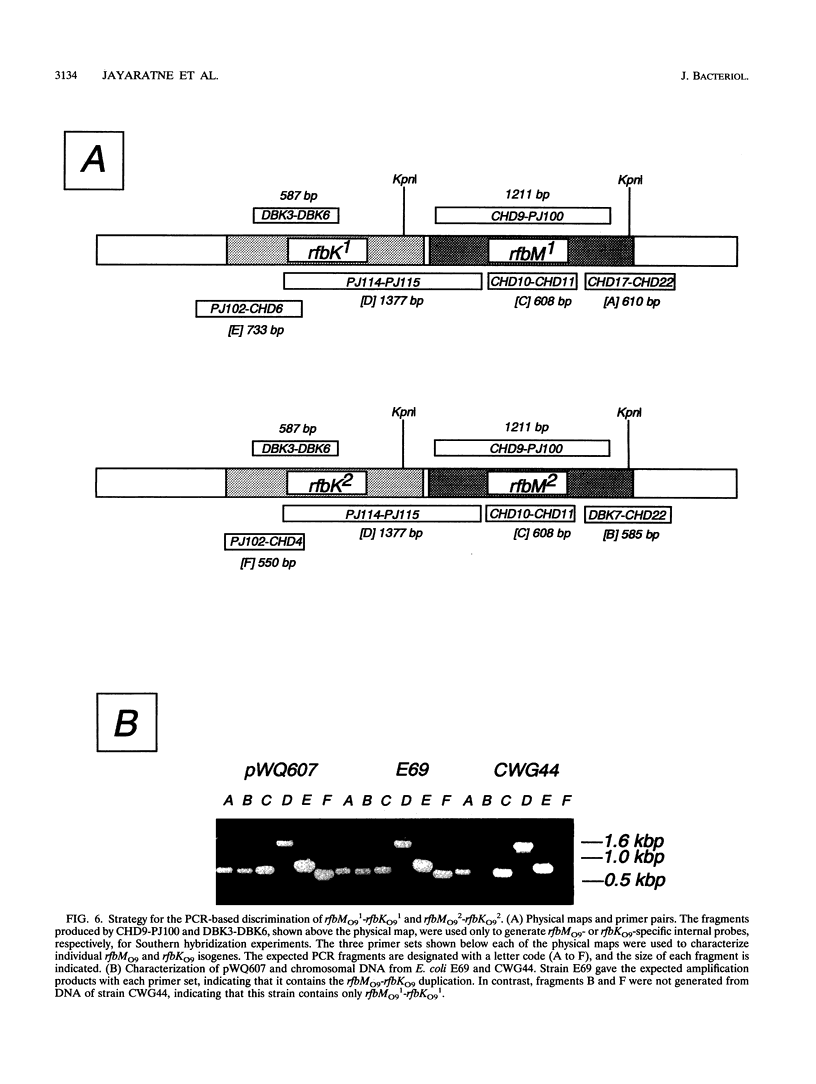
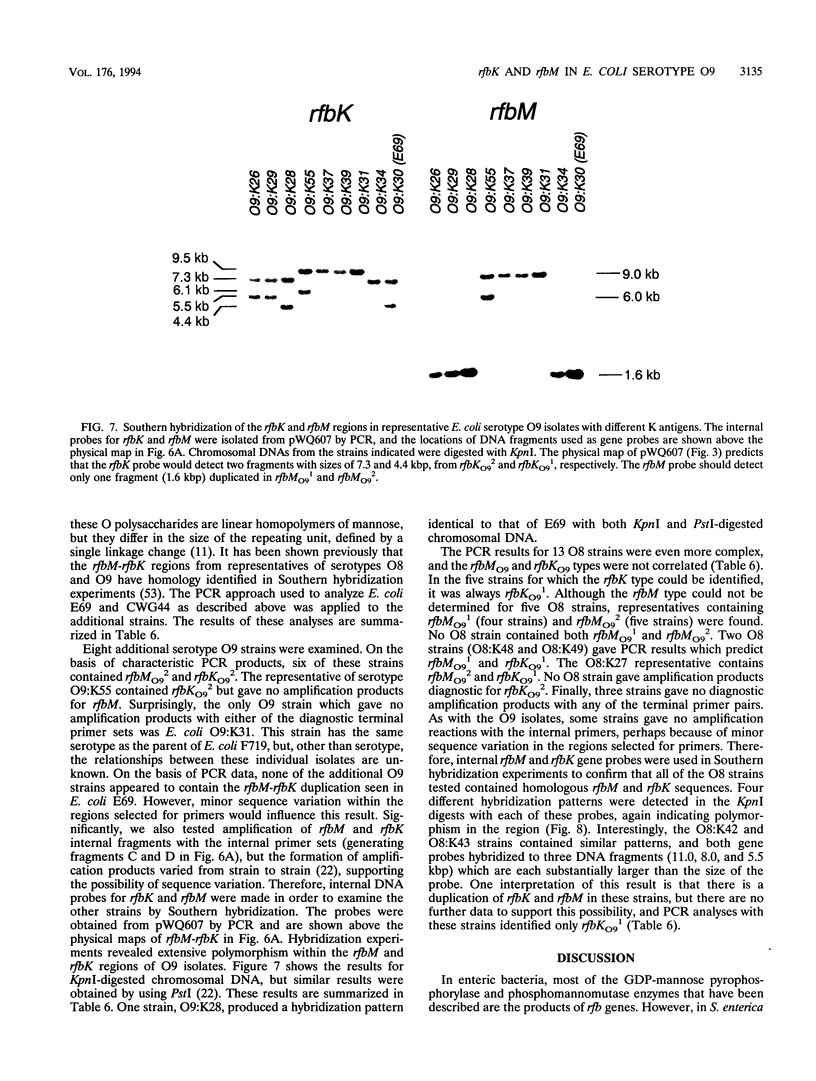
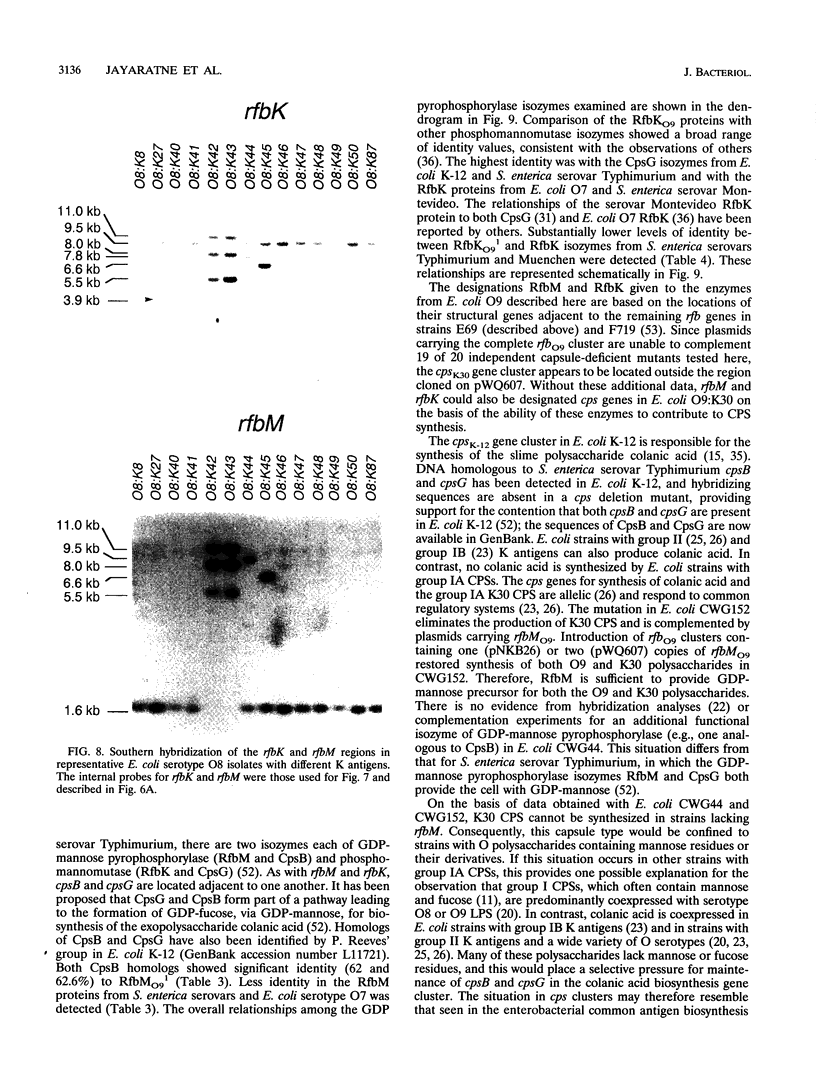
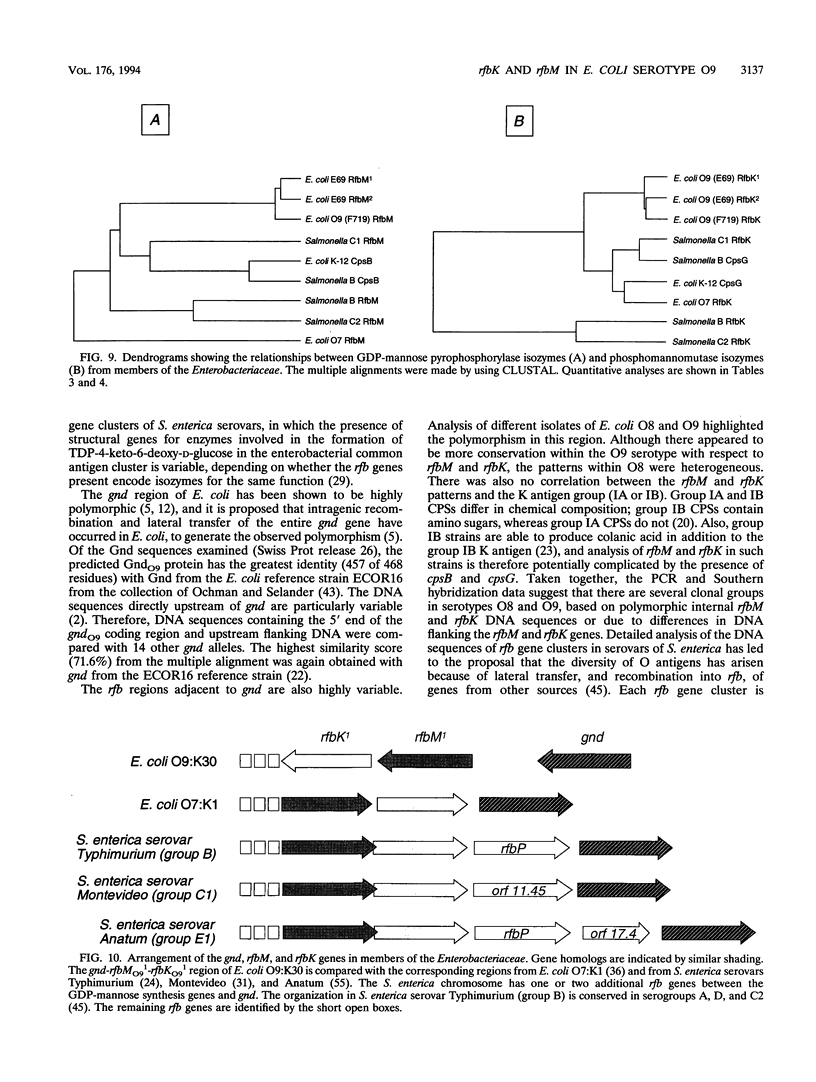
The rfbO9 gene cluster, which is responsible for the synthesis of the lipopolysaccharide O9 antigen, was cloned from Escherichia coli O9:K30. The gnd gene, encoding 6-phosphogluconate dehydrogenase, was identified adjacent to the rfbO9 cluster, and by DNA sequence analysis the gene order gnd-rfbM-rfbK was established. This order differs from that described for other members of the family Enterobacteriaceae. Nucleotide sequence analysis was used to identify the rfbK and rfbM genes, encoding phosphomannomutase and GDP-mannose pyrophosphorylase, respectively. In members of the family Enterobacteriaceae, these enzymes act sequentially to form GDP-mannose, which serves as the activated sugar nucleotide precursor for mannose residues in cell surface polysaccharides. In the E. coli O9:K30 strain, a duplicated rfbM2-rfbK2 region was detected approximately 3 kbp downstream of rfbM1-rfbK1 and adjacent to the remaining genes of the rfbO9 cluster. The rfbM isogenes differed in upstream flanking DNA but were otherwise highly conserved. In contrast, the rfbK isogenes differed in downstream flanking DNA and in 3'-terminal regions, resulting in slight differences in the sizes of the predicted RfbK proteins. RfbMO9 and RfbKO9 are most closely related to CpsB and CpsG, respectively. These are isozymes of GDP-mannose pyrophosphorylase and phosphomannomutase, respectively, which are thought to be involved in the biosynthesis of the slime polysaccharide colanic acid in E. coli K-12 and Salmonella enterica serovar Typhimurium. An E. coli O-:K30 mutant, strain CWG44, lacks rfbM2-rfbK2 and has adjacent essential rfbO9 sequences deleted. The remaining chromosomal genes are therefore sufficient for GDP-mannose formation and K30 capsular polysaccharide synthesis. A mutant of E. coli CWG44, strain CWG152, was found to lack GDP-mannose pyrophosphorylase and lost the ability to synthesize K30 capsular polysaccharide. Wild-type capsular polysaccharide could be restored in CWG152, by transformation with plasmids containing either rfbM1 or rfbM2. Introduction of a complete rfbO9 gene cluster into CWG152 restored synthesis of both O9 and K30 polysaccharides. Consequently, rfbM is sufficient for the biosynthesis of GDP-mannose for both O antigen and capsular polysaccharide E. coli O9:K30. Analysis of a collection of serotype O8 and O9 isolates by Southern hybridization and PCR amplification experiments demonstrated extensive polymorphism in the rfbM-rfbK region.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J. Linkage map of Escherichia coli K-12, edition 8. Microbiol Rev. 1990 Jun;54(2):130–197. doi: 10.1128/mr.54.2.130-197.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barcak G. J., Wolf R. E., Jr Comparative nucleotide sequence analysis of growth-rate-regulated gnd alleles from natural isolates of Escherichia coli and from Salmonella typhimurium LT-2. J Bacteriol. 1988 Jan;170(1):372–379. doi: 10.1128/jb.170.1.372-379.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batchelor R. A., Alifano P., Biffali E., Hull S. I., Hull R. A. Nucleotide sequences of the genes regulating O-polysaccharide antigen chain length (rol) from Escherichia coli and Salmonella typhimurium: protein homology and functional complementation. J Bacteriol. 1992 Aug;174(16):5228–5236. doi: 10.1128/jb.174.16.5228-5236.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binotto J., MacLachlan P. R., Sanderson K. E. Electrotransformation in Salmonella typhimurium LT2. Can J Microbiol. 1991 Jun;37(6):474–477. doi: 10.1139/m91-078. [DOI] [PubMed] [Google Scholar]
- Bisercić M., Feutrier J. Y., Reeves P. R. Nucleotide sequences of the gnd genes from nine natural isolates of Escherichia coli: evidence of intragenic recombination as a contributing factor in the evolution of the polymorphic gnd locus. J Bacteriol. 1991 Jun;173(12):3894–3900. doi: 10.1128/jb.173.12.3894-3900.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar F. Construction and characterization of new cloning vehicles. III. Derivatives of plasmid pBR322 carrying unique Eco RI sites for selection of Eco RI generated recombinant DNA molecules. Gene. 1978 Oct;4(2):121–136. doi: 10.1016/0378-1119(78)90025-2. [DOI] [PubMed] [Google Scholar]
- Brown P. K., Romana L. K., Reeves P. R. Molecular analysis of the rfb gene cluster of Salmonella serovar muenchen (strain M67): the genetic basis of the polymorphism between groups C2 and B. Mol Microbiol. 1992 May;6(10):1385–1394. doi: 10.1111/j.1365-2958.1992.tb00859.x. [DOI] [PubMed] [Google Scholar]
- Clarke B. R., Whitfield C. Molecular cloning of the rfb region of Klebsiella pneumoniae serotype O1:K20: the rfb gene cluster is responsible for synthesis of the D-galactan I O polysaccharide. J Bacteriol. 1992 Jul;174(14):4614–4621. doi: 10.1128/jb.174.14.4614-4621.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darveau R. P., Hancock R. E. Procedure for isolation of bacterial lipopolysaccharides from both smooth and rough Pseudomonas aeruginosa and Salmonella typhimurium strains. J Bacteriol. 1983 Aug;155(2):831–838. doi: 10.1128/jb.155.2.831-838.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditta G., Stanfield S., Corbin D., Helinski D. R. Broad host range DNA cloning system for gram-negative bacteria: construction of a gene bank of Rhizobium meliloti. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7347–7351. doi: 10.1073/pnas.77.12.7347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figurski D. H., Helinski D. R. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1648–1652. doi: 10.1073/pnas.76.4.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman S., Stout V. Regulation of capsular polysaccharide synthesis in Escherichia coli K12. Mol Microbiol. 1991 Jul;5(7):1599–1606. doi: 10.1111/j.1365-2958.1991.tb01906.x. [DOI] [PubMed] [Google Scholar]
- Haraguchi G. E., Zähringer U., Jann B., Jann K., Hull R. A., Hull S. I. Genetic characterization of the O4 polysaccharide gene cluster from Escherichia coli. Microb Pathog. 1991 May;10(5):351–361. doi: 10.1016/0882-4010(91)90080-t. [DOI] [PubMed] [Google Scholar]
- Higgins D. G., Sharp P. M. CLUSTAL: a package for performing multiple sequence alignment on a microcomputer. Gene. 1988 Dec 15;73(1):237–244. doi: 10.1016/0378-1119(88)90330-7. [DOI] [PubMed] [Google Scholar]
- Hitchcock P. J., Brown T. M. Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels. J Bacteriol. 1983 Apr;154(1):269–277. doi: 10.1128/jb.154.1.269-277.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homonylo M. K., Wilmot S. J., Lam J. S., MacDonald L. A., Whitfield C. Monoclonal antibodies against the capsular K antigen of Escherichia coli (O9:K30(A):H12): characterisation and use in analysis of K antigen organisation on the cell surface. Can J Microbiol. 1988 Oct;34(10):1159–1165. doi: 10.1139/m88-204. [DOI] [PubMed] [Google Scholar]
- Jann B., Jann K. Structure and biosynthesis of the capsular antigens of Escherichia coli. Curr Top Microbiol Immunol. 1990;150:19–42. doi: 10.1007/978-3-642-74694-9_2. [DOI] [PubMed] [Google Scholar]
- Jayaratne P., Keenleyside W. J., MacLachlan P. R., Dodgson C., Whitfield C. Characterization of rcsB and rcsC from Escherichia coli O9:K30:H12 and examination of the role of the rcs regulatory system in expression of group I capsular polysaccharides. J Bacteriol. 1993 Sep;175(17):5384–5394. doi: 10.1128/jb.175.17.5384-5394.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X. M., Neal B., Santiago F., Lee S. J., Romana L. K., Reeves P. R. Structure and sequence of the rfb (O antigen) gene cluster of Salmonella serovar typhimurium (strain LT2). Mol Microbiol. 1991 Mar;5(3):695–713. doi: 10.1111/j.1365-2958.1991.tb00741.x. [DOI] [PubMed] [Google Scholar]
- Keenleyside W. J., Bronner D., Jann K., Jann B., Whitfield C. Coexpression of colanic acid and serotype-specific capsular polysaccharides in Escherichia coli strains with group II K antigens. J Bacteriol. 1993 Oct;175(20):6725–6730. doi: 10.1128/jb.175.20.6725-6730.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keenleyside W. J., Jayaratne P., MacLachlan P. R., Whitfield C. The rcsA gene of Escherichia coli O9:K30:H12 is involved in the expression of the serotype-specific group I K (capsular) antigen. J Bacteriol. 1992 Jan;174(1):8–16. doi: 10.1128/jb.174.1.8-16.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knauf V. C., Nester E. W. Wide host range cloning vectors: a cosmid clone bank of an Agrobacterium Ti plasmid. Plasmid. 1982 Jul;8(1):45–54. doi: 10.1016/0147-619x(82)90040-3. [DOI] [PubMed] [Google Scholar]
- Kogan G., Haraguchi G., Hull S. I., Hull R. A., Shashkov A. S., Jann B., Jann K. Structural analysis of O4-reactive polysaccharides from recombinant Escherichia coli. Changes in the O-specific polysaccharide induced by cloning of the rfb genes. Eur J Biochem. 1993 May 15;214(1):259–265. doi: 10.1111/j.1432-1033.1993.tb17919.x. [DOI] [PubMed] [Google Scholar]
- Kuhn H. M., Meier-Dieter U., Mayer H. ECA, the enterobacterial common antigen. FEMS Microbiol Rev. 1988 Sep;4(3):195–222. doi: 10.1111/j.1574-6968.1988.tb02743.x. [DOI] [PubMed] [Google Scholar]
- Laakso D. H., Homonylo M. K., Wilmot S. J., Whitfield C. Transfer and expression of the genetic determinants for O and K antigen synthesis in Escherichia coli O9:K(A)30 and Klebsiella sp. O1:K20, in Escherichia coli K12. Can J Microbiol. 1988 Aug;34(8):987–992. doi: 10.1139/m88-173. [DOI] [PubMed] [Google Scholar]
- Lee S. J., Romana L. K., Reeves P. R. Sequence and structural analysis of the rfb (O antigen) gene cluster from a group C1 Salmonella enterica strain. J Gen Microbiol. 1992 Sep;138(9):1843–1855. doi: 10.1099/00221287-138-9-1843. [DOI] [PubMed] [Google Scholar]
- Liu D., Reeves P. R. Escherichia coli K12 regains its O antigen. Microbiology. 1994 Jan;140(Pt 1):49–57. doi: 10.1099/13500872-140-1-49. [DOI] [PubMed] [Google Scholar]
- Macpherson D. F., Manning P. A., Morona R. Characterization of the dTDP-rhamnose biosynthetic genes encoded in the rfb locus of Shigella flexneri. Mol Microbiol. 1994 Jan;11(2):281–292. doi: 10.1111/j.1365-2958.1994.tb00308.x. [DOI] [PubMed] [Google Scholar]
- Marolda C. L., Valvano M. A. Identification, expression, and DNA sequence of the GDP-mannose biosynthesis genes encoded by the O7 rfb gene cluster of strain VW187 (Escherichia coli O7:K1). J Bacteriol. 1993 Jan;175(1):148–158. doi: 10.1128/jb.175.1.148-158.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCallum K. L., Laakso D. H., Whitfield C. Use of a bacteriophage-encoded glycanase enzyme in the generation of lipopolysaccharide O side chain deficient mutants of Escherichia coli O9:K30 and Klebsiella O1:K20: role of O and K antigens in resistance to complement-mediated serum killing. Can J Microbiol. 1989 Nov;35(11):994–999. doi: 10.1139/m89-166. [DOI] [PubMed] [Google Scholar]
- Neal B. L., Tsiolis G. C., Heuzenroeder M. W., Manning P. A., Reeves P. R. Molecular cloning and expression in Escherichia coli K-12 of chromosomal genes determining the O antigen of an E. coli O2: K1 strain. FEMS Microbiol Lett. 1991 Aug 15;66(3):345–351. doi: 10.1016/0378-1097(91)90286-j. [DOI] [PubMed] [Google Scholar]
- Neuhard J., Thomassen E. Altered deoxyribonucleotide pools in P2 eductants of Escherichia coli K-12 due to deletion of the dcd gene. J Bacteriol. 1976 May;126(2):999–1001. doi: 10.1128/jb.126.2.999-1001.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido H., Levinthal M., Nikaido K., Nakane K. Extended deletions in the histidine-rough-B region of the Salmonella chromosome. Proc Natl Acad Sci U S A. 1967 Jun;57(6):1825–1832. doi: 10.1073/pnas.57.6.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido H., Nikaido K., Mäkelä P. H. Genetic determination of enzymes synthesizing O-specific sugars of Salmonella lipopolysaccharides. J Bacteriol. 1966 Mar;91(3):1126–1135. doi: 10.1128/jb.91.3.1126-1135.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochman H., Selander R. K. Standard reference strains of Escherichia coli from natural populations. J Bacteriol. 1984 Feb;157(2):690–693. doi: 10.1128/jb.157.2.690-693.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyru G., Fraenkel D. G. Genetic mapping of loci for glucose-6-phosphate dehydrogenase, gluconate-6-phosphate dehydrogenase, and gluconate-6-phosphate dehydrase in Escherichia coli. J Bacteriol. 1968 Apr;95(4):1272–1278. doi: 10.1128/jb.95.4.1272-1278.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves P. Evolution of Salmonella O antigen variation by interspecific gene transfer on a large scale. Trends Genet. 1993 Jan;9(1):17–22. doi: 10.1016/0168-9525(93)90067-R. [DOI] [PubMed] [Google Scholar]
- Sanderson K. E., Roth J. R. Linkage map of Salmonella typhimurium, edition VII. Microbiol Rev. 1988 Dec;52(4):485–532. doi: 10.1128/mr.52.4.485-532.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt G., Jann B., Jann K. Genetic and immunochemical studies on Escherichia coli O14:K7:H-. Eur J Biochem. 1974 Feb 15;42(1):303–309. doi: 10.1111/j.1432-1033.1974.tb03340.x. [DOI] [PubMed] [Google Scholar]
- Schmidt G., Jann B., Jann K., Orskov I., Orskov F. Genetic determinants of the synthesis of the polysaccharide capsular antigen K27(A) of Escherichia coli. J Gen Microbiol. 1977 Jun;100(2):355–361. doi: 10.1099/00221287-100-2-355. [DOI] [PubMed] [Google Scholar]
- Shibaev V. N. Biosynthesis of bacterial polysaccharide chains composed of repeating units. Adv Carbohydr Chem Biochem. 1986;44:277–339. doi: 10.1016/s0065-2318(08)60080-3. [DOI] [PubMed] [Google Scholar]
- Stevenson G., Lee S. J., Romana L. K., Reeves P. R. The cps gene cluster of Salmonella strain LT2 includes a second mannose pathway: sequence of two genes and relationship to genes in the rfb gene cluster. Mol Gen Genet. 1991 Jun;227(2):173–180. doi: 10.1007/BF00259668. [DOI] [PubMed] [Google Scholar]
- Sugiyama T., Kido N., Komatsu T., Ohta M., Jann K., Jann B., Saeki A., Kato N. Genetic analysis of Escherichia coli O9 rfb: identification and DNA sequence of phosphomannomutase and GDP-mannose pyrophosphorylase genes. Microbiology. 1994 Jan;140(Pt 1):59–71. doi: 10.1099/13500872-140-1-59. [DOI] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- Wang L., Romana L. K., Reeves P. R. Molecular analysis of a Salmonella enterica group E1 rfb gene cluster: O antigen and the genetic basis of the major polymorphism. Genetics. 1992 Mar;130(3):429–443. doi: 10.1093/genetics/130.3.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield C., Schoenhals G., Graham L. Mutants of Escherichia coli O9:K30 with altered synthesis and expression of the capsular K30 antigen. J Gen Microbiol. 1989 Oct;135(10):2589–2599. doi: 10.1099/00221287-135-10-2589. [DOI] [PubMed] [Google Scholar]
- Wilkinson R. G., Gemski P., Jr, Stocker B. A. Non-smooth mutants of Salmonella typhimurium: differentiation by phage sensitivity and genetic mapping. J Gen Microbiol. 1972 May;70(3):527–554. doi: 10.1099/00221287-70-3-527. [DOI] [PubMed] [Google Scholar]
- Wolf R. E., Jr, Shea F. M. Combined use of strain construction and affinity chromatography in the rapid, high-yield purification of 6-phosphogluconate dehydrogenase from Escherichia coli. J Bacteriol. 1979 Apr;138(1):171–175. doi: 10.1128/jb.138.1.171-175.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]