Abstract
HIV-1 infection of the central nervous system (CNS) frequently causes dementia and other neurological disorders. The mechanisms of CNS injury in HIV-1 infection are poorly understood. Apoptosis of neurons and astrocytes is induced by HIV-1 infection in vitro and in brain tissue from AIDS patients, but the apoptotic stimuli have not been identified. We report herein that HIV-1 infection of primary brain cultures induces the receptor tyrosine kinase protooncogene c-kit and that high levels of c-Kit expression are associated with astrocyte apoptosis. Overexpression of c-Kit in an astrocyte-derived cell line in the absence of HIV-1 induces rapid apoptotic death. The apoptotic mechanism requires the c-Kit tyrosine kinase domain. The mechanism of c-kit induction by HIV-1 involves transactivation of the c-kit promoter by the HIV-1 Nef protein. These studies demonstrate that c-Kit can induce astrocyte apoptosis and suggest that this mechanism may play a role in CNS injury caused by HIV-1 infection. We propose that c-Kit can serve dual functions as a growth factor receptor or apoptosis inducer.
HIV-1 infection of the central nervous system (CNS) frequently causes AIDS dementia and other neurological disorders (1). The major target cells for HIV-1 infection in the brain are macrophages and microglia (1–3). Astrocytes are also infected, but only at a low level due to restricted HIV-1 gene expression (4–6). The mechanisms of CNS injury in HIV-1 infection are poorly understood. Neurons are not directly infected by HIV-1, suggesting that neuronal loss is caused by indirect mechanisms. Several factors have been proposed to contribute to CNS injury caused by HIV-1 infection. These include soluble forms of the HIV-1 gp120 and Tat proteins and factors secreted by HIV-1-infected macrophages and microglia, such as tumor necrosis factor α, platelet activating factor, oxygen free radicals, nitric oxide, and excitatory amino acids (7). However, the in vivo role of these factors in contributing to CNS injury has not been established.
Apoptosis of neurons and astrocytes is induced by HIV-1 infection in vitro (8) and in brain tissue from AIDS patients (8–11), but the apoptotic stimuli have not been identified. Apoptosis plays a critical role during CNS development but in the adult brain is only associated with pathological conditions such as stroke and Alzheimer disease. To identify candidate genes for apoptosis inducers in HIV-1 infection of the CNS, we used differential display (12) to identify induced mRNAs in HIV-1-infected primary brain cultures compared with uninfected cultures. We report herein that HIV-1 infection of primary brain cultures induces the protooncogene c-kit. Our studies demonstrate that overexpression of c-Kit can induce astrocyte apoptosis and provide evidence that this mechanism may play a role in CNS injury caused by HIV-1 infection.
METHODS
Cell Culture and HIV-1 Infection.
Primary human brain cultures were prepared from fetal abortuses (13–18 weeks), plated in 24-well plates (200,000–250,000 cells per well), and maintained in DMEM containing 10% calf serum for 10–20 days prior to infection as described (8, 13). Tissue was procured in accordance with institutional regulations. These cultures contain a mixture of astrocytes (70–90%), neurons (10–30%), microglial cells (1–5%), and fibroblasts (1–5%) (8). Infection with HIV-189.6 (14) was performed by incubation with 100,000 reverse transcriptase (RT) units of virus stock for 16 h at 37°C. A 50% medium change was performed every 4–7 days. Productive HIV-1 infection was confirmed by monitoring RT activity in the culture supernatants every 5–7 days (8). The human astrocyte-derived U87 and human embryonic kidney fibroblast 293 cell lines were maintained in DMEM with 10% fetal calf serum.
Plasmids.
The c-Kit expression plasmid pcKit was constructed by subcloning the human c-kit DNA (15) into pCDNA3 (Invitrogen). The c-Kit deletion mutants were made in pcKit using the ExSite PCR-based site-directed mutagenesis kit (Stratagene). The following oligonucleotides were used: ΔED, 5′-TGTCTGGACGCGAAGCAGTAGGAG-3′ and 5′-CTCTTCACTCCTTTGCTG-3′; ΔID, 5′-CAGAATCATCACAATAATGCACATCATGC CAGC-3′ and 5′-TTGGCCAACTGCAGCCCCAA-3′; ΔTKDI, 5′-GGGAAACTCCCATTTGTGATC-3′ and 5′-AGAAAACGTGATTCATTCATATGTTCAAAGCA-3′; ΔTKDII, 5′-GGCCAACTCGTCATCCTCCATGAT-3′ and 5′-TTGGCCAACTGCAGCCCCAA-3′; ΔTKD, 5′-GGGAAACTCCCATTTGTGATC-3′ and 5′-TTGGCCAACTGCAGCCCCAA-3′; ΔABM, 5′-GGTTTTCCCAAAACTCAGCC-3′ and 5′-GTTGAGGCAACTGCATATGG-3′. The mutants were confirmed by dideoxynucleotide DNA sequencing. The pCD161 chloramphenicol acetyltransferase (CAT) reporter gene plasmid contains the human c-kit promoter (positions −20 to −2094) (16) subcloned into a Bluescript II (Stratagene) bacterial CAT reporter gene plasmid. The NL4–3, SG3.1, and YU2 plasmids encode full-length HIV-1 proviral DNAs (17, 18). The Nef(Eli) and Nef(Bru) plasmids encode nef alleles of the Eli and Bru HIV isolates (19, 20). The 239nef and YEnef plasmids encode pathogenic and nonpathogenic simian immunodeficiency SIV nef alleles (21). The HXE-Eli, -Yu10, and -YU21 plasmids encode full-length HXB2 HIV-1 proviral DNA containing nef alleles derived from the Eli, YU10, or YU21 HIV-1 isolates (19). HXE-Eli.ΔXhoI contains a deletion of Eli nef (19).
Differential Display.
Differential display was performed as described (12), with the following modifications. Total RNA was isolated by using the RNA Prep kit (Oncor) and treated with RNase-free DNase (Boehringer Mannheim) to remove residual genomic DNA. cDNA synthesis was performed with T12VM primer (Operon Technologies, Alameda, CA) and Superscript II RNase H-RT (GIBCO/BRL). One-tenth of the cDNA synthesis reaction products was amplified by PCR with AmpliTaq (Perkin-Elmer Cetus) in the presence of 35S-labeled dATP by using different combinations of arbitrary 10-mers in conjunction with anchored oligo(dT) primers (Operon) for 40 cycles at 94°C for 45 sec, 40°C for 90 sec, and 72°C for 30 sec. The 35S-labeled PCR products were denatured, resolved on 8% DNA sequencing gels, and subcloned into pCRII (TA Cloning Kit, Invitrogen) for DNA sequencing.
RT-Coupled PCR (RT–PCR) and Northern Blot Analysis.
For RT–PCR analysis, total RNA was isolated as described above and 0.2 μg was used for cDNA synthesis using T12VM primer and Superscript II RNase H-RT (GIBCO/BRL). One-tenth of this reaction product was used for PCR amplification (c-kit primers, 5′-AGGGGAAAACACCATAAG-3′ and 5′-GGAACAGGTACAGTCAGATGAG-3′; β-actin primers, 5′-CAGGTCATCACCATT GGCAATGAG-3′ and 5′-CAGCACTGTGTTGCGTACAGGTC-3′) for 40 cycles at 94°C for 1 min, 55°C for 1 min, and 72°C for 1 min. For Northern blot analysis, c-kit mRNA was detected by probing with the 32P-labeled random-primed (Boehringer Mannheim) 662-bp RT–PCR product obtained with c-kit primers.
Western Blot Analysis.
Cells were lysed in 50 mM Tris·HCl, pH 8.0/2 mM EDTA/1% Nonidet P-40/137 mM NaCl/10% glycerol/2 mM Na3VO4/100 μM leupeptin/1 mM phenylmethylsulfonyl fluoride. One milligram of protein was immunoprecipitated with rabbit anti-c-Kit (Oncogene Science) and analyzed by Western blotting with the same antibody by using the ECL system (Amersham).
Immunofluorescence Staining.
Cells were fixed in 4% paraformaldehyde in phosphate-buffered saline (PBS) for 30 min. Double immunofluorescence staining with primary antibodies followed by fluorescein isothiocyanate- or rhodamine-secondary antibodies (Sigma) was performed as described (8). The dilutions for the primary antibodies were as follows: mouse anti-c-Kit monoclonal, 1:50 (PharMingen); rabbit anti-glial fibrillary acidic protein, 1:500 (Sigma); rabbit anti-Nef, 1:1,000 (19).
Detection of Apoptosis.
Terminal deoxynucleotidyltransferase-mediated dUTP nick end-labeling (TUNEL) staining was performed with the Apoptag kit (Oncor). Hoechst 33342 staining (1 μg/ml for 10 min at 37°C) was used to detect apoptotic nuclear morphology. Propidium iodide staining was performed by incubation of a fixed cell suspension in 3 mM sodium citrate buffer (pH 7.0) containing propidium iodide at 50 μg/ml and 0.1% Triton X-100 for 1 h at 37°C. The percentage of apoptotic cells was determined by propidium iodide staining and counting the number of nuclei with morphologic features characteristic of apoptosis (chromatin condensation and nuclear fragmentation) by using a ×20 objective. For analysis of DNA fragmentation on agarose gels, soluble cytoplasmic DNA fragments were isolated as described (22), separated by electrophoresis in 1% agarose gels, blotted onto Hybond-N membrane (Amersham), and hybridized with 32P-labeled EcoRI-digested human genomic DNA as a probe.
RESULTS
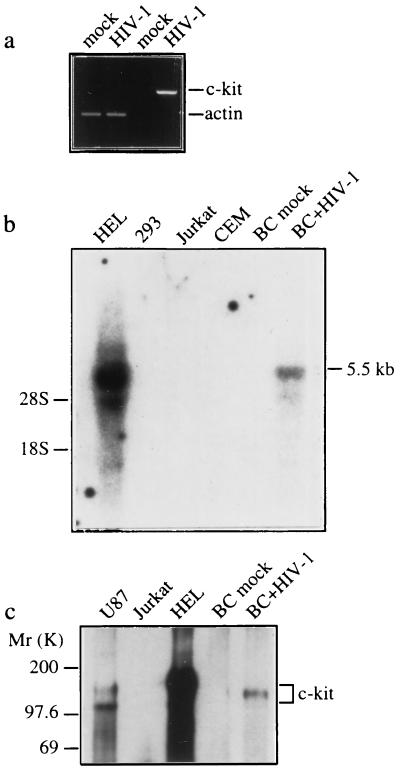
To identify candidate genes for apoptosis inducers in HIV-1 infection of the CNS, we used differential display (12) to identify induced mRNAs in a panel of cDNAs derived from HIV-1-infected primary human fetal brain cells compared with uninfected cells. Primary brain cultures were infected with HIV-189.6 and total RNA was isolated on day 30 after infection. We have demonstrated (8) that apoptosis of neurons and astrocytes is significantly induced by HIV-189.6 infection at this time. A 216-bp partial cDNA induced by HIV-1 infection was identified with the 5′ primer TTCCGAACCC and 3′ primer TTTTTTTTTTTTVA (where V is an equimolar mixture of dA, dC, and dG). Dideoxynucleotide DNA sequencing and a GenBank search showed that the partial cDNA was identical to the human protooncogene c-kit, which encodes a transmembrane receptor tyrosine kinase that binds stem cell factor (23, 24). Marked induction of c-kit mRNA and protein in HIV-1-infected primary brain cultures was demonstrated by RT–PCR, Northern blot, and Western blot analyses (Fig. 1 a–c). In control cultures, c-kit expression was very low or undetectable.
Figure 1.
Induction of c-kit by HIV-1 infection is associated with astrocyte apoptosis. Primary human fetal brain cultures (BC) were infected with HIV-189.6 or mock-infected, and c-Kit expression was examined by RT–PCR (a), Northern blot (b), and Western blot (c) analyses and immunofluorescence staining (d–f) at 30 days after infection. Endogenous c-Kit is detected in HEL and U87 cells but not in Jurkat, CEM, and 293 cells (b and c). (a) RT–PCR analysis of c-kit and β-actin transcripts. (b) Northern blot analysis of c-kit mRNA (5.5 kb) in total RNA (30 μg) isolated from mock- and HIV-1-infected brain cultures. The positions of 18S and 28S ribosomal RNA are indicated. (c) Western blot analysis of c-Kit (145 kDa). The two forms of c-Kit detected in U87 cells most likely correspond to the 120-kDa precursor and 145-kDa mature forms (25). (d–f) Double immunofluorescence staining of HIV-1-infected cultures with the primary antibodies indicated followed by fluorescein isothiocyanate- or rhodamine-secondary antibodies. (d) c-Kit colocalizes with the astrocyte-specific marker anti-glial fibrillary acidic protein. (e) Apoptosis in c-Kit-positive cells detected by TUNEL staining (arrows). (f) Apoptotic nuclear morphology in c-Kit-positive Nef-positive astrocyte (arrows) detected by nuclear staining with Hoechst 33342. (Left) Lower row is a double exposure. Cultures shown in f were treated with recombinant tumor necrosis factor α (10 ng/ml) for 48 h, which was necessary to permit detection of Nef in astrocytes (26). Treatment with tumor necrosis factor α did not change the expression of c-Kit or the relative number of apoptotic astrocytes in mock- versus HIV-1-infected cultures. Original magnifications: ×200 (d), ×300 (e), and ×400 (f). Results in a–f were similar in two or three experiments.
The c-Kit receptor mediates diverse physiological responses, including cell proliferation, differentiation, and survival. c-Kit is normally expressed in hematopoietic cells, mast cells, melanocytes, and germ cells and is essential for normal hematopoiesis, melanogenesis, and gametogenesis (27). c-Kit is also expressed in astrocytes and some neuronal subpopulations in the CNS, where its normal function and role in development have not been defined (24, 27, 28). We investigated which cell populations in HIV-1-infected primary brain cultures showed induction of c-Kit to determine whether there was a correlation between infected cells and c-Kit expression. The induction of c-Kit in HIV-1-infected brain cultures occurred exclusively in astrocytes (Fig. 1 d–f). c-Kit staining did not colocalize with neuron- or microglial-specific markers and was not detected in mock-infected cultures (data not shown). The HIV-1 Nef protein has been used as a marker for HIV-1-infected astrocytes, because Gag and Env expression are prevented by a cellular block in Rev function (4, 5, 26, 29). Overexpression of endogenous c-Kit was detected in HIV-1 Nef-positive astrocytes (Fig. 1f). High levels of endogenous c-Kit in this astrocyte subpopulation were associated with a marked increase in the frequency of apoptosis as determined by TUNEL staining to detect DNA fragmentation and Hoechst 33342 staining to detect apoptotic nuclear morphology (Fig. 1f). The frequency of apoptosis in c-Kit-positive HIV-1 Nef-positive astrocytes (Fig. 1f) was 25 ± 3% versus 1.2 ± 0.2% of astrocytes in mock-infected cultures (mean ± SEM, n = 3, P < 0.01 by Student’s t test). The frequency of apoptosis in the total c-Kit-positive astrocyte population in HIV-1-infected cultures (Nef-positive and Nef-negative; Fig. 1e) was 2.2 ± 0.4% versus 0.7 ± 0.2% of astrocytes in mock-infected cultures (mean ± SEM, n = 3, P < 0.05). These results demonstrate that HIV-1 infection results in marked induction of c-Kit and provide evidence that overexpression of endogenous c-Kit is associated with astrocyte apoptosis.
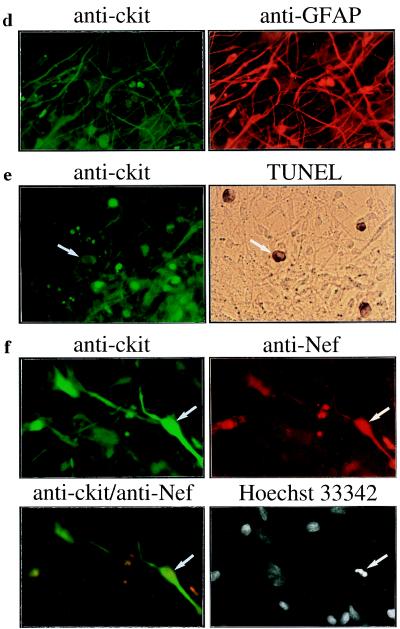
To investigate the link between c-Kit overexpression and astrocyte apoptosis, we transfected the astrocyte-derived cell line U87, which expresses endogenous c-Kit (30) (Fig. 1 b and c), with the c-Kit expression plasmid pcKit. The c-kit transfected cells began to show loss of adherence and evidence of cell death at 24 h after transfection, and cell viability was reduced to 10% of control levels by 48 h (Fig. 2a). Cell death in the c-kit transfected cultures was due to apoptosis, which was induced in a time-dependent manner and correlated directly with the amount of transfected c-kit DNA (Fig. 2 b–f). However, apoptosis was not induced by overexpression of c-Kit in transfected 293, COS-1, or Hela cells, which do not express endogenous c-Kit (data not shown). Thus, induction of apoptosis by c-Kit is cell-type-specific. These results indicate that overexpression of c-Kit is sufficient to induce astrocyte apoptosis in the absence of HIV-1.
Figure 2.
Apoptosis induced by overexpression of c-Kit. U87 cells (5 × 105 cells) were transfected with 2.5 μg or the indicated amount of pcKit (○) or the pcDNA3 vector (•) by lipofection with N-[1-(2,3-dioleoyl)propyl]-N,N,N-trimethylammonium methyl sulfate (DOTAP; Boehringer Mannheim). The transfection efficiency was 80–90% as determined by transfection with pCMV-βgal. (a) Cell survival was determined by counting the number of cells that excluded trypan blue. (b) The percentage of apoptotic cells was determined by analysis of nuclear morphology after staining with propidium iodide. (c and d) Analysis of DNA fragmentation on agarose gels. Soluble cytoplasmic DNA was isolated from cells transfected with pcKit at the indicated time points (c) or 48 h (d) after transfection. Apoptosis induced by staurosporine (0.1 mM for 16 h) gave the same pattern of DNA fragmentation as that shown in c and d, lanes 4–6 (data not shown). (e) Hoechst 33342 staining demonstrates apoptotic nuclei (arrows) in cells transfected with pcKit but not the control vector pCDNA3. (f) Apoptotic nuclei in c-Kit-positive cells (solid arrows) demonstrated by Hoechst 33342 staining. A normal nucleus is indicated by the open arrow. For e and f, cells were fixed at 48 h after transfection. Data in a and b are the means ± SD for duplicate determinations. Results shown are representative of at least two experiments.
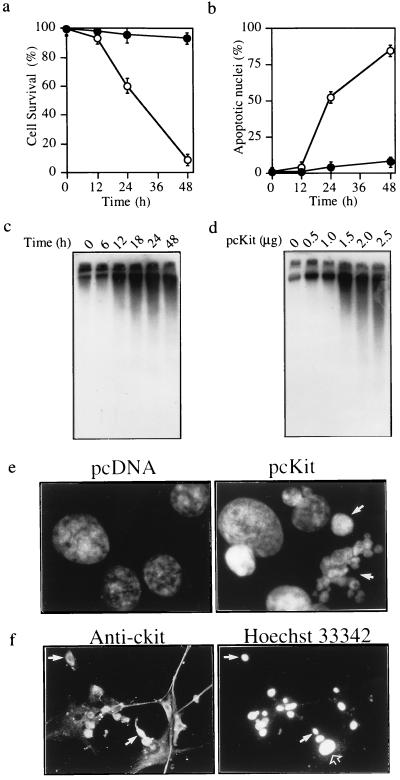
We then examined which domains of c-Kit are required for induction of apoptosis. The c-Kit receptor is related to the platelet-derived growth factor receptor family and the macrophage colony-stimulating factor receptor, which constitute type III receptor tyrosine kinases (RTKs) (23, 24, 27). The unique features of this RTK subclass are an extracellular ligand-binding domain that contains five Ig-like repeats and an intracellular tyrosine kinase domain that is split by a hydrophilic kinase insert domain (27). Based on sequence homologies and studies of other RTK subclass III receptors, we constructed a series of deletion mutants to determine which region(s) of c-Kit are required for induction of apoptosis (Fig. 3a). Mutant c-Kit proteins of the predicted relative molecular masses were expressed at levels similar to wild type, except for the ΔTKDII mutant, which was expressed at lower levels (Fig. 3b). Transfection of the c-kit mutants into U87 cells showed that deletion of the extracellular domain, intracellular domain, tyrosine kinase domain II, or entire tyrosine kinase domain resulted in loss of apoptosis induction (Fig. 3 c and d). Deletion of tyrosine kinase domain I or the ATP-binding motif had no significant effect. Thus, the extracellular domain and an intact tyrosine kinase domain are both required for induction of apoptosis by c-Kit.
Figure 3.
Induction of apoptosis by c-Kit requires an intact tyrosine kinase domain. (a) Schematic diagram of c-Kit deletion mutants (ΔED, extracellular domain; ΔID, intracellular domain; ΔABM, ATP-binding motif ΔTKDI, tyrosine kinase domain I; ΔTKDII, tyrosine kinase domain II; ΔTKD, entire tyrosine kinase domain). (b) Expression of c-Kit mutants. Each plasmid at 20 μg was transfected into 293 cells by the calcium phosphate method. Immunoprecipitation of 500 μg of cellular protein followed by Western blotting with anti-c-Kit was performed at 48 h after transfection. (c and d) Induction of apoptosis by c-Kit mutants. Each plasmid at 2.5 μg was transfected into U87 cells as in Fig. 2. The cytoplasmic DNA fragmentation assay (c) and quantitation of apoptosis (d) were performed at 48 h after transfection as in Fig. 2 b–d. Data in d are the means ± SD for duplicate determinations. Results were similar in two or three experiments.
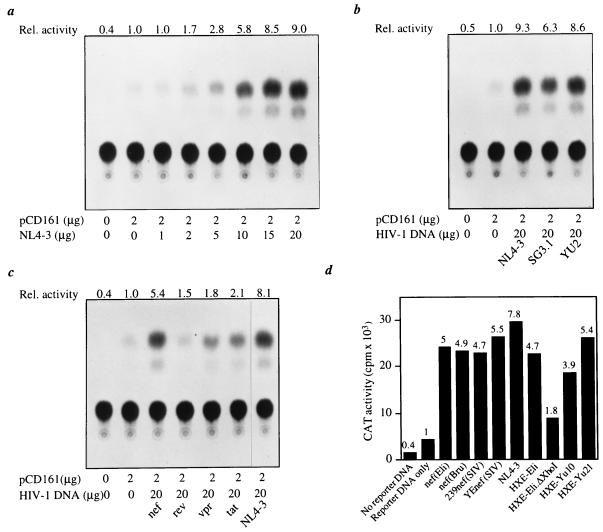
We then investigated the mechanism of c-kit induction by HIV-1. To determine whether HIV-1 transactivates the c-kit promoter, the HIV-1 NL4–3 proviral DNA was transfected into 293 cells with the CAT reporter construct pCD161 driven by the human c-kit promoter. HIV-1 NL4–3 transactivated CAT expression up to 9-fold, an activity that correlated directly with the amount of cotransfected HIV-1 DNA (Fig. 4a). Similar results were obtained using HIV-1 proviral DNAs from three different viral isolates (Fig. 4b). We then determined which HIV-1 gene products were responsible for the transactivation activity. Cotransfection of HIV-1 Nef, Tat, Vpr, or Rev expression plasmids with the pCD161 reporter construct showed that Nef induced CAT activity by approximately 6-fold (Fig. 4c). In contrast, Tat, Vpr, and Rev showed only weak transactivation activity (1.5- to 2-fold) (Fig. 4c). To further test the transactivation activity of Nef, we cotransfected pCD161 with different HIV-1 or SIV Nef expression plasmids or HIV-1 proviral DNAs bearing different HIV-1 nef alleles (Fig. 4d). The transactivation activity of Nef was similar regardless of the nef allele tested. There was no significant difference in transactivation activity between pathogenic and nonpathogenic SIV nef alleles or between nef alleles of brain-derived (YU10 and YU21) and non-brain-derived (Eli and Bru) HIV-1 isolates (Fig. 4d). The deletion of nef in the HXE-Eli.ΔXhoI provirus significantly reduced transactivation activity compared with its wild-type counterpart HXE-Eli (Fig. 4d). These results demonstrate that Nef is responsible for most of the HIV-1 transactivation activity and provide an explanation for the high level of c-Kit induction and apoptosis in Nef-positive astrocytes.
Figure 4.
HIV-1 transactivation of the c-kit promoter. Transcriptional activation in 293 cells was determined by measuring CAT activity of the reporter construct pCD161 driven by the human c-kit promoter. 293 cells were transfected with 2 μg of CD161 and the indicated amounts (a–c) or 20 μg (d) of plasmid encoding full-length HIV-1 proviral DNA (NL4–3, SG3.1, YU2, or HXE) or HIV-1 Nef, Rev, Vpr, or Tat expression plasmids. Cell lysates prepared 48 h after transfection were assayed for CAT activity (20). All determinations were normalized to the activity of cotransfected pCMV-βgal. (a) pCD161 was cotransfected with different amounts of pNL4–3 HIV-1 proviral DNA. (b–d) Transactivation of the c-kit promoter by different HIV-1 isolates (b), HIV-1 proteins (c), or nef alleles (d). The Nef(Eli) plasmid was used in c. The relative activity above the basal level is shown at the top of each thin layer chromatography plate or bar graph. In d, the cpm were from converted acetylated chloramphenicol. Results shown are from single experiments that were repeated two or three times with similar results.
DISCUSSION
These studies demonstrate that c-Kit can induce astrocyte apoptosis and provide evidence that this mechanism may play a role in CNS injury caused by HIV-1 infection. Our studies show that HIV-1 infection of primary brain cultures results in marked induction of c-Kit and that overexpression of endogenous c-Kit is associated with astrocyte apoptosis. Moreover, we demonstrate that overexpression of c-Kit in an astrocyte-derived cell line is sufficient to induce apoptosis in the absence of HIV-1. Astrocyte cell death may contribute indirectly to neuronal injury or other CNS pathologies associated with HIV-1 infection (1, 8, 9), since astrocytes provide neurotrophic support, protect against excitatory amino acid neurotoxicity, and maintain the normal homeostasis of the extracellular fluid.
Our data suggest that the mechanism of c-kit induction by HIV-1 involves transactivation of the c-kit promoter by HIV-1 Nef. The c-kit promotor contains potential binding sites for Sp1, Myb, AP-2, basic helix–loop–helix proteins, Ets family proteins, and GATA-1 (16, 31). However, little is known about the mechanisms that activate c-kit transcription. The true function of Nef is unknown. Nef enhances HIV-1 replication but has not been shown to directly activate transcription. Effects of Nef include down-regulation of CD4, alteration of T cell activation pathways, and enhancement of virion infectivity (for review, see ref. 32). It is possible that Nef may activate the c-kit promoter by an indirect mechanism, such as altered signaling due to its interactions with cellular kinases (21, 33, 34).
Our mutagenesis studies suggest that induction of apoptosis by c-Kit is mediated by signaling through its kinase domain. c-Kit is normally activated by binding to its ligand, which induces receptor dimerization, tyrosine autophosphorylation, enhanced tyrosine kinase activity, and interaction with specific src-homology 2 domain-containing signaling molecules. However, we found that stem cell factor (100 ng/ml) failed to enhance or prevent apoptosis induced by c-Kit overexpression in transfected U87 cells (J.H. and D.G., unpublished data), suggesting that apoptosis induction in this system occurs by a ligand-independent mechanism. One possibility is that the high levels of c-Kit in transfected U87 cells induce constitutive receptor dimerization and activation. This would be consistent with our finding that the extracellular domain, which contains the dimerization signal, and the kinase domain are both required for induction of apoptosis. Deletion of the ATP binding domain did not result in loss of apoptosis induction. However, this finding may reflect transphosphorylation between endogenous and mutant c-Kit receptors within heterologous dimers (25, 27).
The demonstration that c-Kit can serve dual functions as both a growth factor receptor and apoptosis inducer has implications for understanding the diverse biological roles of growth factor receptors. The c-Kit ligand prevents apoptosis in hematopoietic cells, mast cells, and natural killer cells (35–37). These findings and our results suggest that c-Kit can both cause and prevent cell death. This dual phenotype of c-Kit may be shared with other type III RTKs, since the platelet-derived growth factor receptor has been shown to induce apoptosis in growth-arrested cells (38). Similarly, a recent study demonstrated that the nerve growth factor receptor p75 can both cause and prevent apoptotic cell death during normal development (39). The induction of apoptosis by certain growth factor receptors is a mechanism that may regulate cell number during development or normal tissue homeostasis and may be subverted by certain pathological stimuli such as HIV-1 infection. Whether the signal is directed to cell growth or apoptosis may depend on factors such as the ability of cells to progress through the cell cycle, the presence of other growth factors, or the coexpression of specific signaling molecules (38). Understanding these mechanisms may provide insights that are relevant to therapies for AIDS and other diseases.
Acknowledgments
We thank Dr. B. Yankner for discussion and providing primary brain cultures; Dr. B. Hahn, Dr. G. Shaw, and Dr. S. Ghosh for the YU-2.C and SG3.1 plasmids; Dr. R. Desrosiers for the SIV Nef plasmids; and Dr. E. Zazopoulos for the HIV-1 Nef plasmids. The pNL4–3 plasmid (donated by Dr. M. Martin) and HIV-189.6 isolate (donated by Dr. R. Collman) were obtained from the National Institutes of Health AIDS Research and Reference Reagent Program. This work was supported by Pediatric AIDS Foundation 50565-18-PG and National Institutes of Health Grant NS35734 and by gifts from the G. Harold and Leila Y. Mathers Charitable Foundation and the Friends 10. We acknowledge the Center for AIDS Research (AI28691) and Center for Cancer Research (AO6514) grants for supporting necessary core facilities.
ABBREVIATIONS
- CNS
central nervous system
- TUNEL
terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling
- RTK
receptor tyrosine kinase
- CAT
chloramphenicol acetyltransferase
- RT
reverse transcriptase
- RT–PCR
RT-coupled PCR
- SIV
simian immunodeficiency virus
References
- 1.Price R W, Brew B, Sidtis J, Rosenblum M, Scheck A C, Cleary P. Science. 1988;239:586–591. doi: 10.1126/science.3277272. [DOI] [PubMed] [Google Scholar]
- 2.Gabuzda D H, Ho D D, de la Monte S M, Rota T R, Sobel R A. Ann Neurol. 1986;20:289–295. doi: 10.1002/ana.410200304. [DOI] [PubMed] [Google Scholar]
- 3.Gartner S, Markovits P, Markovits D M, Kaplan M H, Gallo R C, Popovic M. Science. 1986;233:214–218. doi: 10.1126/science.3014648. [DOI] [PubMed] [Google Scholar]
- 4.Saito Y, Sharer L R, Epstein L G, Michaels J, Mintz M, Louder M, Golding K, Cvetkovich T A, Blumberg B M. Neurology. 1994;44:474–481. doi: 10.1212/wnl.44.3_part_1.474. [DOI] [PubMed] [Google Scholar]
- 5.Tornatore C, Chandra R, Berger J R, Major E O. Neurology. 1991;44:481–487. doi: 10.1212/wnl.44.3_part_1.481. [DOI] [PubMed] [Google Scholar]
- 6.Takahashi K, Wesselingh S L, Griffin D E, McArthur J C, Johnson R T, Glass J D. Ann Neurol. 1996;39:705–711. doi: 10.1002/ana.410390606. [DOI] [PubMed] [Google Scholar]
- 7.Lipton S A, Gendelman H E. N Engl J Med. 1995;332:934–940. doi: 10.1056/NEJM199504063321407. [DOI] [PubMed] [Google Scholar]
- 8.Shi B, De Girolami U, He J, Wang S, Lorenzo A, Busciglio J, Gabuzda D. J Clin Invest. 1996;98:1979–1990. doi: 10.1172/JCI119002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Petito C K, Roberts B. Am J Pathol. 1995;146:1121–1130. [PMC free article] [PubMed] [Google Scholar]
- 10.Adie-Biasette H, Levy Y, Colombel M, Poron F, Natchev S, Keohane C, Gray F. Neuropathol Appl Neurobiol. 1995;21:218–227. doi: 10.1111/j.1365-2990.1995.tb01053.x. [DOI] [PubMed] [Google Scholar]
- 11.Gelbard H A, James H J, Sharer L R, Perry S W, Saito Y, Kazee A M, Blumberg B M, Epstein L G. Neuropathol Appl Neurobiol. 1995;21:208–217. doi: 10.1111/j.1365-2990.1995.tb01052.x. [DOI] [PubMed] [Google Scholar]
- 12.Liang P, Pardee A B. Science. 1992;257:967–971. doi: 10.1126/science.1354393. [DOI] [PubMed] [Google Scholar]
- 13.Busciglio J, Yeh J, Yankner B A. J Neurochem. 1993;61:1565–1568. doi: 10.1111/j.1471-4159.1993.tb13658.x. [DOI] [PubMed] [Google Scholar]
- 14.Collman R, Balliet J W, Gregory S A, Friedman H, Kolson D L, Nathanson N, Srinivasan A. J Virol. 1992;66:7517–7521. doi: 10.1128/jvi.66.12.7517-7521.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vandenbark G R, deCastro C M, Taylor H, Dew-Knight S, Kaufman R E. Oncogene. 1992;7:1259–1266. [PubMed] [Google Scholar]
- 16.Vandenbark G R, Chen U, Friday E, Pavlik K, Anthony B, deCastro C, Kaufman R E. Cell Growth Differ. 1996;7:1383–1392. [PubMed] [Google Scholar]
- 17.Ghosh S K, Fultz P N, Keddie E, Saag M S, Sharp P M, Hahn B H, Shaw G M. Virology. 1993;194:858–864. doi: 10.1006/viro.1993.1331. [DOI] [PubMed] [Google Scholar]
- 18.Li Y, Hui H, Burgess C J, Price R W, Sharp P M, Hahn B M, Shaw G M. J Virol. 1992;66:6587–6600. doi: 10.1128/jvi.66.11.6587-6600.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zazopoulos E, Haseltine W. Virology. 1993;194:20–27. doi: 10.1006/viro.1993.1230. [DOI] [PubMed] [Google Scholar]
- 20.Zazopoulos E, Haseltine W A. Proc Natl Acad Sci USA. 1992;89:6634–6638. doi: 10.1073/pnas.89.14.6634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Du Z, Lang S M, Sasseville V G, Lackner A A, Ilyinskii P O, Daniel M D, Jung J U, Desrosiers R C. Cell. 1995;82:665–674. doi: 10.1016/0092-8674(95)90038-1. [DOI] [PubMed] [Google Scholar]
- 22.Herrmann M, Lorenz H-M, Voll R, Grünke M, Woith W, Kalden J R. Nucleic Acids Res. 1994;22:5506–5507. doi: 10.1093/nar/22.24.5506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yarden Y, Kuang W, Yang-Feng T, Coussens L, Munemitsu S, Dull T J, Chen E, Schlessinger J, Franke U, Ullrich A. EMBO J. 1987;6:3341–3351. doi: 10.1002/j.1460-2075.1987.tb02655.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qiu F, Ray P, Brown K, Barker P E, Jhanwar S, Ruddle F H, Besmer P. EMBO J. 1988;7:1003–1011. doi: 10.1002/j.1460-2075.1988.tb02907.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blume-Jensen P, Claesson-Welsh L, Siegbahn A, Zsebo K M, Westermark B, Heldin C-H. EMBO J. 1991;10:4121–4128. doi: 10.1002/j.1460-2075.1991.tb04989.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tornatore C, Meyers K, Atwood W, Conant K, Major E. J Virol. 1994;68:93–102. doi: 10.1128/jvi.68.1.93-102.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lev S, Blechman J M, Givol D, Yarden Y. Crit Rev Oncog. 1994;5:141–168. doi: 10.1615/critrevoncog.v5.i2-3.30. [DOI] [PubMed] [Google Scholar]
- 28.Natali P G, Nicotra M R, Sures I, Santoro E, Bigotti A, Ullrich A. Cancer Res. 1992;52:6139–6143. [PubMed] [Google Scholar]
- 29.Neumann M, Felber B K, Kleinschmidt A, Froese B, Erfle V, Pavlakis G N, Brack-Warner R. J Virol. 1995;69:2159–2167. doi: 10.1128/jvi.69.4.2159-2167.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stanulla M, Welte K, Hadam M R, Pietsch T. Acta Neuropathol. 1995;89:158–165. doi: 10.1007/BF00296360. [DOI] [PubMed] [Google Scholar]
- 31.Yamamoto K, Tojo A, Aoki N, Shibuya M. Jpn J Cancer Res. 1993;84:1136–1144. doi: 10.1111/j.1349-7006.1993.tb02813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Trono D. Cell. 1995;82:189–192. doi: 10.1016/0092-8674(95)90306-2. [DOI] [PubMed] [Google Scholar]
- 33.Sawai E T, Baur A, Struble H, Peterlin B M, Levy J A, Cheng-Mayer C. Proc Natl Acad Sci USA. 1994;91:1539–1543. doi: 10.1073/pnas.91.4.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saksela K, Cheng G, Baltimore D. EMBO J. 1995;14:484–491. doi: 10.1002/j.1460-2075.1995.tb07024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Caceres-Cortés J, Rajotte D, Dumouchel J, Haddad P, Hoang T. J Biol Chem. 1994;269:12084–12091. [PubMed] [Google Scholar]
- 36.Yee N S, Paek I, Besmer P. J Exp Med. 1994;179:1777–1787. doi: 10.1084/jem.179.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carson W E, Haldar S, Baiocchi R A, Croce C M, Caligiuri M A. Proc Natl Acad Sci USA. 1994;91:7553–7557. doi: 10.1073/pnas.91.16.7553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim H-R C, Upadhyay S, Li G, Palmer K C, Deuel T F. Proc Natl Acad Sci USA. 1995;92:9500–9504. doi: 10.1073/pnas.92.21.9500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Frade J M, Rodríguez-Tébar, Barde Y-A. Nature (London) 1996;383:166–168. doi: 10.1038/383166a0. [DOI] [PubMed] [Google Scholar]