Abstract
Rotavirus infection is the leading cause of severe diarrhea in infants and young children worldwide. The rotavirus nonstructural protein NSP4 acts as a viral enterotoxin to induce diarrhea and causes Ca2+-dependent transepithelial Cl− secretion in young mice. The cellular basis of this phenomenon was investigated in an in vitro cell line model for the human intestine. Intracellular calcium concentration ([Ca2+]i) was monitored in fura-2-loaded HT-29 cells using microscope-based fluorescence imaging. NSP4 (1 nM to 5 μM) induced both Ca2+ release from intracellular stores and plasmalemma Ca2+ influx. During NSP4-induced [Ca2+]i mobilization, [Na+]i homeostasis was not disrupted, demonstrating that NSP4 selectively regulated extracellular Ca2+ entry into these cells. The ED50 of the NSP4 effect on peak [Ca2+]i mobilization was 4.6 ± 0.8 nM. Pretreatment of cells with either 2.3 × 10−3 units/ml trypsin or 4.4 × 10−2 units/ml chymotrypsin for 1–10 min abolished the NSP4-induced [Ca2+]i mobilization. Superfusing cells with U-73122, an inhibitor of phospholipase C, ablated the NSP4 response. NSP4 induced a rapid onset and transient stimulation of inositol 1,4,5-trisphosphate (IP3) production in an IP3-specific radioreceptor assay. Taken together, these results suggest that NSP4 mobilizes [Ca2+]i in human intestinal cells through receptor-mediated phospholipase C activation and IP3 production.
Keywords: calcium mobilization, diarrhea, HT-29 cells, NSP4, rotavirus enterotoxin
Rotaviruses cause severe gastroenteritis in young children and animals. These triple-layered viruses contain a genome of 11 segments of double-stranded RNA that codes for six structural and five nonstructural proteins. Rotaviruses have a unique morphogenic pathway that involves budding of immature particles through the membrane of the endoplasmic reticulum (ER) in host cells (1, 2). The nonstructural protein NSP4 (encoded by rotavirus gene 10) functions as an intracellular receptor on the ER membrane for immature particles to acquire the outer capsid protein layer (3, 4).
Calcium has been shown to be an important factor for the oligomerization of NSP4 in the ER and stabilization of the outer capsid protein layer (5). Ca2+ homeostasis is altered in rotavirus-infected MA104 cells and has been correlated with rotavirus-specific protein synthesis (6, 7). Michelangeli and coworkers have suggested that the intracellular calcium concentration ([Ca2+]i) increases observed in virus-infected cells result from the failure of the cellular Ca2+-homeostatic processes to compensate for increased Ca2+ entry promoted by a viral product (6). Furthermore, accumulation of the viral product and its concomitant effect on [Ca2+]i mobilization may be responsible for cytotoxicity and cell death during the later stages of viral infection (7). Our laboratory has reported that the rotavirus nonstructural protein NSP4 increases [Ca2+]i in Sf9 insect cells when expressed endogenously or added exogenously (8), and that NSP4 induces diarrhea in young mice when injected intraperitoneally or intraileally (9). In addition, we have suggested that NSP4 may be a candidate for the viral product proposed by Michelangeli and coworkers and that NSP4-induced [Ca2+]i mobilization may be responsible for some of the cellular aspects of rotavirus pathogenesis. However, [Ca2+]i mobilization by NSP4 has not been demonstrated in human intestinal cells, and the cellular mechanisms by which NSP4 may signal alterations in [Ca2+]i within enterocytes remain unknown.
Based on our earlier in vitro and in vivo findings in insect cells and the small intestinal mucosa of mice, we have hypothesized two ways by which NSP4 mobilizes [Ca2+]i in host cells: (i) NSP4 synthesized in virus-infected cells may release [Ca2+]i by interacting with ER Ca2+ pools; and (ii) after being released from virus-infected cells, NSP4 may bind to an unidentified molecule on or within the plasma membrane of uninfected neighboring cells to mobilize [Ca2+]i. The existence of two mechanisms to mobilize [Ca2+]i has been demonstrated in insect cells (8), but the details of the signaling pathways are not yet understood. The [Ca2+]i increase generated by either mechanism could stimulate endogenous fluid secretory pathways within the intestinal mucosa and cause the early onset (<4 h) secretory diarrhea observed in animal models. In this study, we have investigated whether NSP4 can mobilize [Ca2+]i in uninfected human intestinal cells.
For these studies, we used the human colonic adenocarcinoma cell line HT-29 clone 19A (10) as an in vitro model to investigate how NSP4 causes Ca2+-dependent Cl− secretion across the mammalian small intestinal mucosa (9). This cell line expresses marker proteins found in both the small and large intestine, including dipeptidyl peptidase IV (11), the neurotensin receptor (12–14), the M3 muscarinic receptor (15), and the vasoactive intestinal peptide receptor (16). Receptor occupancy by phospholipase C (PLC)-dependent agonists in HT-29 cells leads to the activation of phosphatidylinositol turnover (17), [Ca2+]i mobilization (18–21), and monolayer Cl− secretion (21, 22). In addition, HT-29 cells can be infected with the simian rotavirus SA11 strain (23). This study shows that NSP4 induces [Ca2+]i mobilization in HT-29 cells by stimulating both intracellular Ca2+ release and extracellular Ca2+ influx controlled by a mechanism involving PLC activation and inositol 1,4,5-trisphosphate (IP3) production.
MATERIALS AND METHODS
Reagents.
The TRK 1000 competitive radioreceptor assay was purchased from Amersham. Fura-2/AM and sodium-binding benzofuran isophthalate acetoxymethyl ester (SBFI/AM) were purchased from Molecular Probes. The TransPort Kit, a transient and reversible membrane permeabilization product, was obtained from GIBCO. Aminosteroid U-73122 and U-73343 were obtained from Calbiochem. Trypsin-TPCK and chymotrypsin were purchased from Worthington. Neurotensin, neomycin, and all other reagents were acquired from Sigma.
The standard bath solution for Ca2+ imaging and Na+ imaging was Na-Hepes-buffered physiological saline, containing 140 mM NaCl, 4.7 mM KCl, 1.13 mM MgCl2, 10 mM Hepes, 10 mM glucose, and 1 mM CaCl2. The solution for neomycin loading by the TransPort Kit was K-Hepes-buffered physiological saline, containing 80 mM potassium glutamate, 40 mM KCl, 20 mM NaCl, 1.15 mM MgCl2, 10 mM Hepes, 10 mM glucose, and 0.1 mM EGTA (pH 7.2), which mimics the intracellular milieu (31).
Cell Culture.
HT-29 clone 19A cells (10) were routinely cultured in DMEM with 4.5 g/liter glucose, supplemented with 4 mM l-glutamine/100 units/ml penicillin/streptomycin/5 μg/ml gentamicin/10% fetal bovine serum. Passages between 25 and 40 were used.
NSP4 Purification.
NSP4 was purified from insect Sf9 cells infected with a recombinant baculovirus pAC461-G10 expressing rotavirus gene 10 (encoding NSP4). Cells were harvested 96 h after infection in Hink’s medium and lysed with lysis buffer (10 mM Tris·HCl, pH 8.1/0.1 mM EDTA/1% CHAPS). NSP4 was first semipurified by fast protein liquid chromatography using a quaternary methylamine anion exchange column (Waters) preequilibrated with equilibration buffer (20 mM Tris·HCl, pH 8.1). The NSP4-rich fractions were pooled for further purification using an agarose immunoaffinity column onto which purified rabbit IgG against NSP4114–135 had been immobilized (9, 24). The bound NSP4 was eluted with 0.1 M Tris·HCl buffer at pH 2.8. The eluate was then dialyzed against 50 mM NH4HCO3 and lyophilized. NSP4 was freshly dissolved in PBS before use.
Fura-2/AM Loading.
Confluent monolayers of HT-29 cells were trypsinized, and 0.33 ml of cells (5 × 104/ml) was seeded onto the center of glass coverslips in cloning cylinders (0.2 cm2 × 0.8 cm) and incubated at 37°C in DMEM for 24 h. These coverslips were then glued onto experimental chambers and incubated with Na-Hepes-buffered saline containing 10 μM fura-2/AM for 45–60 min at room temperature. They were then superfused continuously with Na-Hepes (with 1 mM Ca2+) for 15 min at 37°C to remove extracellular dye and cleave any remaining intracellular AM-ester dye forms. The bath temperature was maintained at 37°C by prewarming the extracellular solutions and by water-jacketing the oil immersion lens of the inverted microscope.
[Ca2+]i Imaging.
All experiments were carried out with the aid of a high resolution camera imaging system as described previously (21). In brief, light emitted from fura-2-loaded cells at 510 nm was captured by an intensified video camera (Intensifier, Videoscope International, Sterling, VA, optically coupled to a Dage Newvicon tube camera, MTI Inc., Michigan City, IN) following exposure to both 340- and 380-nm excitation light. The camera signal was then digitally encoded using a Matrox image-capture board and processed using image analysis software (image1/fl, Universal Imaging, Media, PA). The background subtracted images were ratioed on a pixel-by-pixel basis to yield a bitmap field. Calibration of the fura-2 dye fluorescence was carried out using the ionophore ionomycin under Ca2+-free and Ca2+-saturating conditions as described previously (21), and the [Ca2+]i was calculated according to the Grynkiewicz equation (25). The [Ca2+]i values of individual field pixels obtained by this procedure were color-coded using a color look-up table and displayed on an RGB monitor and stored on the hard disk. Six to 12 cells from each camera field were chosen for time dependent analysis of cellular [Ca2+]i. The averaged ratio signal obtained from each cell was digitally saved as a log file. The collected values from cells imaged within a single experiment were then averaged together to give an experimental observation of 1 (n = 1). Between 3 and 17 separate experimental observations from different dye loadings were routinely collected for each experimental condition.
[Na+]i Imaging.
Cells were loaded with the fluorescent sodium indicator SBFI/AM as described by Minta and Tsien (26). [Na+]i imaging was performed using the experimental setup described for [Ca2+]i imaging. Intracellular Na+ levels were calibrated by adding a 1 μM concentration of the ion channel-forming pentadecapeptide gramicidin to cells bathed with 1–140 mM Na/K-Hepes-buffered saline (27, 28). For all experiments, proteins and peptides were superfused onto cells by a glass pipette and a microinjection system (picosprizer, General Valve, Fairfield, NJ).
Measurement of Intracellular IP3.
The IP3 assay was performed as previously described (17, 18, 29). Briefly, confluent HT-29 cells were trypsinized and plated (107 per well) on 12-well plates and incubated at 37°C for 24 h. At 1 day after seeding, the cells were preincubated for 30 min at 37°C with 20 mM LiCl (to inhibit inositol-1-phosphatase). The cells were then incubated in 500 μl of Na-Hepes-buffered saline containing 1 mM Ca2+ and treated with either NSP4 or neurotensin to achieve a final concentration of 100 nM and 50 nM, respectively. The reactions were terminated at various time points by aspiration of the solution, addition of 500 μl of 4% (vol/vol) perchloric acid, and incubation for 20 min on ice. After centrifugation and neutralization with 10 M KOH, aliquots of the supernatant were assayed for IP3 by a competitive radioreceptor assay (Amersham) performed according to the manufacturer’s instruction (29).
Neomycin Loading.
HT-29 cells seeded on coverslips were washed three times with K-Hepes-buffered saline and then incubated for 6 min at 37°C in 250 μl K-Hepes-buffered saline containing 100 μM fura-2 free acid, with or without 100 μM neomycin, and 50 μl of the permeating solution from the TransPort Kit (30, 31). Permeabilization was stopped by aspiration of the permeating solution and incubation for 5 min at 37°C in 250 μl of fresh K-Hepes-buffered saline containing 100 μl of the stop solution from the kit. Cells were then superfused in Na-Hepes-buffered saline containing 1 mM CaCl2 for the experiments.
RESULTS
NSP4 Transiently Increased [Ca2+]i in HT-29 Cells.
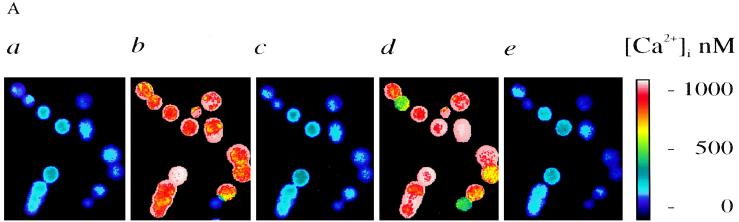
The exogenous addition of NSP4 to Sf9 insect cells was shown to increase [Ca2+]i (8). In the present study, NSP4 effects on [Ca2+]i in human intestinal cells were investigated by single-cell fluorescence imaging. The addition of 50 nM NSP4 to HT-29 cells elicited a rapid onset (within seconds) and transient [Ca2+]i increase that lasted for approximately 1–2 min (Fig. 1A). Images were taken from one representative experiment (Fig. 1 Aa–Ac). The resting [Ca2+]i in HT-29 cells was 79 ± 9 nM (mean ± SEM, n = 15), and the peak response to NSP4 ([Ca2+]i peak) was 684 ± 111 nM (n = 15). The transient response suggests that a receptor-mediated process involving either intracellular Ca2+ release and/or extracellular Ca2+ influx was responsible for the NSP4 effect. Nominally Ca2+-free extracellular solution did not affect the magnitude of the NSP4 response (n = 4; data not shown), confirming that the major source of the transient [Ca2+]i rise induced by NSP4 came from intracellular Ca2+ store release.
Figure 1.
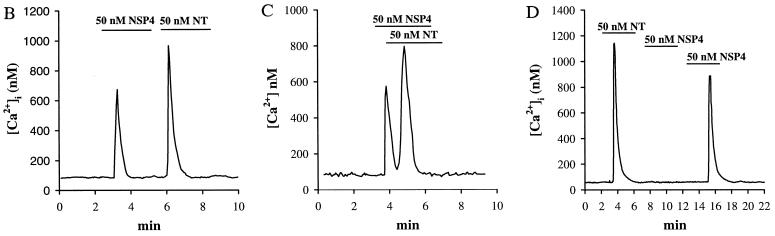
Effects of NSP4 on [Ca2+]i in fura-2-loaded HT-29 cells. (A) Images taken from a representative experiment illustrating [Ca2+]i values after consecutive superfusion of 50 nM NSP4 and 50 nM neurotensin. [Ca2+]i was imaged before the onset of stimulation by NSP4 (0 s; a), during the peak response to NSP4 (15 s; b), after the [Ca2+]i returned to the resting levels (80 s; c), during the peak response to neurotensin (260 s; d), and after the [Ca2+]i returned to the resting levels (360 s; e). Calibrated [Ca2+]i values were displayed on a pseudocolor scale. (B and C) Time-dependent tracings of average [Ca2+]i values recorded during a single experiment after the consecutive superfusion of 50 nM NSP4 and 50 nM neurotensin (NT). Both agonists were superfused for 3 min as indicated by the bar. The traces were the average [Ca2+]i recorded from 10 cells, representing 15 separate experiments (n = 15) in B and 3 separate experiments (n = 3) in C. (D) 50 nM NSP4 was added to the cells after addition of 50 nM neurotensin (n = 3).
HT-29 cells respond to the neuropeptide neurotensin with a transient increase in [Ca2+]i mediated by phosphatidylinositol turnover (18–21). Neurotensin at 50 nM was used as a positive control in the following studies. Neurotensin (50 nM) induced a [Ca2+]i increase in HT-29 cells with kinetics similar to those recorded for NSP4, except that the mean of the neurotensin peak response was larger (1,085 ± 86 nM, n = 15), and the duration of the response to neurotensin was longer (30 ± 10 s; Fig. 1 Ad and Ae, and B–D). When 50 nM neurotensin was superfused onto cells after the addition of 50 nM NSP4, the cells always responded to neurotensin regardless of the length of interval between the NSP4 and neurotensin additions (n = 15; Fig. 1B). If neurotensin was added immediately after the peak response to NSP4, the [Ca2+]i values would first return to resting levels and then immediately increase again in response to the neurotensin receptor occupancy (Fig. 1C). This phenomenon is consistent with previous documentation that neurotensin increases [Ca2+]i by mobilizing IP3-sensitive internal Ca2+ stores which must be refilled after depletion before a second [Ca2+]i release can be achieved (18–20). The recovery time needed for the cells to respond to neurotensin after NSP4 challenge was 18 ± 6 s (n = 3; Fig. 1C). In contrast, when 50 nM NSP4 was superfused onto the cells after an initial neurotensin-induced [Ca2+]i mobilization, it took 13 ± 1 min for the cells to subsequently elicit a second [Ca2+]i response (n = 3; Fig. 1D). This observation suggests that NSP4 uses overlapping internal Ca2+ stores that take longer to refill than those mobilized by neurotensin.
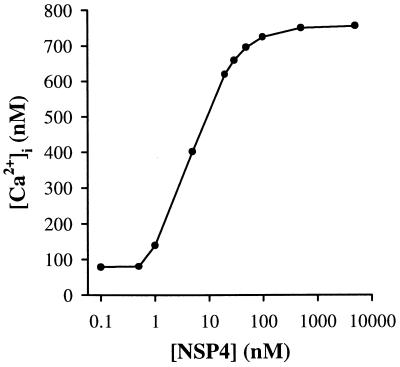
The [Ca2+]i dose–response curve of HT-29 cells challenged with NSP4 (Fig. 2) showed a saturable hyperbolic function with a calculated ED50 of 4.6 ± 0.8 nM, and the maximum [Ca2+]i response (750 ± 107 nM) at a NSP4 concentration of 100 nM (n = 4). We therefore used 50 nM NSP4 in most of our experiments, which gave 91% of the maximum [Ca2+]i response (684 ± 111 nM, n = 15). The saturable hyperbolic relationship of the NSP4 dose–response suggests that an enzyme-catalyzed process is involved.
Figure 2.
Dose–response relationship of NSP4-induced [Ca2+]i peak increase in HT-29 cells. The data were curve-fitted to a sigmoid logistic function using non-linear regression (Sigma plot, Jandel Scientific, Corte Madera, CA). The calculated ED50 value was 4.6 ± 0.8 nM. The NSP4 concentrations used were 0.1, 0.5, 1, 5, 20, 30, 50, 100, 500, and 5,000 nM. For each data point, n = 4.
NSP4 Elicited a Ca2+ Influx in HT-29 Cells.
The PLC-dependent receptor agonists can maintain a sustained [Ca2+]i mobilization response after intracellular Ca2+ release by inducing extracellular Ca2+ influx in HT-29 cells (19, 30, 31), which is important for Ca2+-store refilling (32). We therefore examined whether a similar extracellular Ca2+ influx component could be observed during the NSP4-induced [Ca2+]i mobilization. This was achieved by using the fura-2 fluorescence-quenching properties of the divalent cation Mn2+ (26). Mn2+ enters epithelial cells by the same route as Ca2+ (33, 34). Hence, Mn2+ quench of the fura-2 fluorescence signal can be used to monitor Ca2+ influx across the plasma membrane. In practice, the fluorescence quench by Mn2+ at either 340- or 380-nm excitation wavelengths can be masked by the significant fluorescence changes that occur after agonist-mediated Ca2+ release. An alternative and more accurate method is to monitor Mn2+ quenching at the 360-nm excitation wavelength where fura-2 dye emission is independent of [Ca2+]i (19). As shown in Fig. 3, when HT-29 cells excited at 360 ± 10 nm were superfused in Na-Hepes-buffered saline (nominally Ca2+-free) containing 250 μM MnCl2, there was a slight decrease in the fluorescence signal due to quenching of the residual extracellular dye and the low resting permeability of the plasma membrane to divalent cations. Superfusing 50 nM NSP4 caused a large and slightly delayed (27 ± 6 s) fluorescence quench (n = 4), indicating that Ca2+ influx was indeed occurring secondarily to the initial phase of NSP4-induced [Ca2+]i release. NSP4 stimulated a 6.5 ± 1.5-fold increase in the rate of Ca2+ influx compared with basal rates (n = 4).
Figure 3.
NSP4 induces a Ca2+ influx in HT-29 cells. Fura-2-loaded cells excited with 360-nm light were superfused with Na-Hepes saline (without Ca2+) containing 250 μM MnCl2. NSP4 was added onto cells as indicated (n = 17).
To further demonstrate that NSP4 induces Ca2+ influx exclusively through an alteration in plasma membrane Ca2+ ion permeability, we investigated whether [Na+]i homeostasis was disrupted during the period of NSP4-induced [Ca2+]i mobilization. Cells were loaded with SBFI/AM and [Na+]i was visualized at the same dual excitation and single emission wavelengths as those employed for [Ca2+]i imaging. Addition of 50–500 nM NSP4 onto SBFI-loaded cells had little or no effect on the resting [Na+]i levels (12.6 ± 2.0 mM, n = 3; data not shown), whereas the divalent cationic ionophore ionomycin elicited a large Na+ influx ([Na+]i peak = 22.5 ± 3.0 mM, n = 3; data not shown). Increases in [Na+]i elicited by ionomycin can be explained by high (>1 μM) [Ca 2+]i levels which can subsequently activate nonspecific cation channels within the plasma membrane leading to extracellular Na+ influx (30). These results further confirm that NSP4 acts to regulate the plasma membrane Ca2+ permeability and not the permeability of other cations.
Pretreatment of Cells with Proteases Eliminated the NSP4 [Ca2+]i Response.
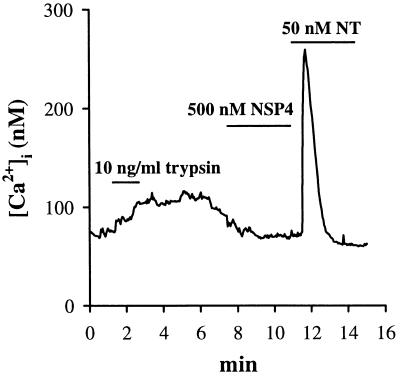
To examine if NSP4 exerts its effect on [Ca2+]i mobilization through interactions with plasma membrane-bound proteins, we treated the cells with the protease trypsin before addition of NSP4. Cells superfused with 2.3 × 10−3 units/ml (10 ng/ml) trypsin for 1 min lost their response to NSP4 ([Ca2+]i peak = 86 ± 11 nM, n = 6; Fig. 4; doses up to 500 nM NSP4 were used) and gave attenuated responses to neurotensin ([Ca2+]i peak = 221 ± 66 nM, n = 6; Fig. 4). Note that the [Ca2+]i was slightly elevated upon exposure to trypsin ([Ca2+]i peak = 102 ± 9 nM, n = 6). This later observation may be correlated with the finding that nanomolar concentrations of trypsin can activate a protease-activated receptor 2 (PAR-2), leading to inositol phospholipid hydrolysis and [Ca2+]i mobilization (35). This receptor has been shown to be expressed at high levels in the human pancreas, liver, kidney, small intestine, and colon (35). The cells were also pretreated with chymotrypsin, a protease which does not signal through the PAR-2 receptor, to rule out the possibility that PAR-2 receptor activation was masking the NSP4 effect by depleting the same intracellular Ca2+ stores. A 10-min pretreatment with 4.4 × 10−2 units/ml (1 μg/ml) chymotrypsin abolished the [Ca2+]i rise elicited by 50 nM NSP4 and significantly attenuated the [Ca2+]i mobilization elicited by both 500 nM NSP4 and 50 nM neurotensin (n = 3; data not shown). Chymotrypsin at a concentration of 4.4 × 10−3 units/ml had no effect on NSP4 or neurotensin-induced [Ca2+]i mobilization. These findings indicate that a plasma membrane-bound receptor with a protease sensitivity profile different from that of the neurotensin receptor may initiate NSP4-induced [Ca2+]i mobilization.
Figure 4.
Pretreatment of cells with trypsin either abolished (n = 6) or attenuated (n = 6) the [Ca2+]i mobilization elicited by 100 nM NSP4 or 50 nM neurotensin, respectively. Cells were superfused with Na-Hepes-buffered saline containing 2.3 × 10−3 units/ml (10 ng/ml) trypsin for 1 min, then washed for 5 min in protease-free saline before addition of either agonist.
Inhibition of PLC Abolished the NSP4 Effect on [Ca2+]i Mobilization.
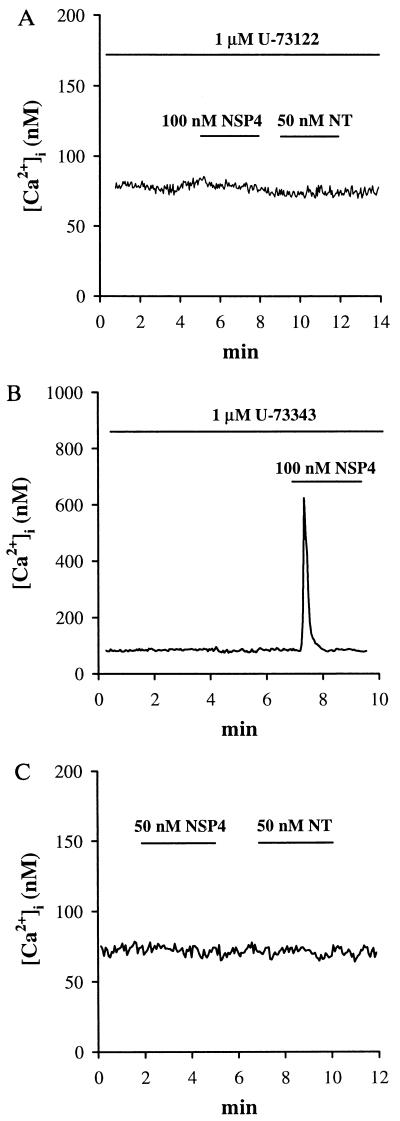
Phosphatidylinositol 4,5-bisphosphate (PIP2) is hydrolyzed by PLC in response to the activation of G protein-coupled receptors to yield two important cellular second messengers: sn(1,2) diacylglycerol (DAG) and d-myo-inositol 1,4,5-trisphosphate (IP3) (36). PLC-catalyzed hydrolysis of PIP2 can be inhibited by the aminosteriod U-73122, which subsequently prevents the formation of both DAG and IP3 and abolishes IP3-dependent [Ca2+]i mobilization in cells (37, 38). A close analog of this molecule, U-73343 has no inhibitory effects on PLC-dependent cellular signaling in cells and is commonly used as a control. These two analogs were separately superfused onto fura-2-loaded cells for 5 min before the addition of either NSP4 or neurotensin. U-73122 (1 μM) abolished the [Ca2+]i mobilization by both 50 nM NSP4 and 50 nM neurotensin ([Ca2+]i peak = 80 ± 4 and 79 ± 4 nM, respectively; n = 3; Fig. 5A). In contrast, 1 μM U-73343 had no effect on the [Ca2+]i mobilization induced by either agonist ([Ca2+]i peak = 625 ± 83 and 988 ± 76 nM, respectively; n = 3; Fig. 5B; neurotensin response not shown).
Figure 5.
Inhibition of PLC abolished the NSP4-induced [Ca2+]i rise. (A) Cells were superfused with 1 μM U-73122 for 5 min. NSP4 and neurotensin were added onto cells as indicated (n = 3). (B) Cells were superfused for 5 min with 1 μM U-73343, an inactive analog of U-73122. NSP4 was added onto cells as indicated (n = 3). (C) Cells preloaded with 100 μM fura-2 free acid and 100 μM neomycin were challenged with NSP4 and neurotensin consecutively (n = 3).
Neomycin binds to phosphoinositide and inhibits phosphoinositide hydrolysis by PLC (39, 40). Neomycin (100 μM) and 100 μM fura-2 free acid were dissolved in the K-Hepes-buffered saline previously shown to maintain both [Ca2+]i and plasma membrane ion channel responsiveness to neurotensin after plasma membrane permeabilization and resealing (31). Both the NSP4 and the neurotensin effects were abolished using this procedure ([Ca2+]i peak = 75 ± 6 and 75 ± 5 nM, respectively; n = 3; Fig. 5C). Omitting neomycin from the loading buffer maintained normal responses in HT-29 cells to both agonists ([Ca2+]i peak = 665 ± 79 and 1026 ± 97 nM, respectively; n = 3; data not shown). These results show that PLC activation is necessary for NSP4-induced [Ca2+]i mobilization in these cells.
NSP4 Stimulated IP3 Production in HT-29 Cells.
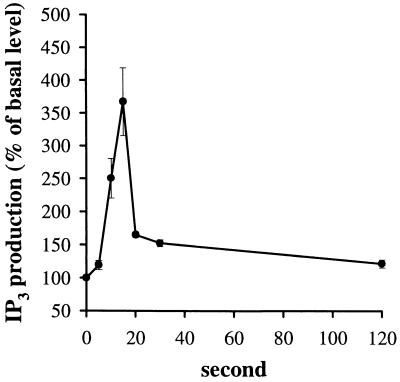
Agonists that mobilize [Ca2+]i by signaling through PLC activation stimulate the production of IP3 (36). Elevated levels of IP3 then bind to the IP3 receptors on the ER membrane to elicit the release of stored Ca2+. The next question we pursued in our identification of the cellular signaling pathway for NSP4 was whether NSP4 stimulated phosphoinositide hydrolysis could be detected in human intestinal cells. We performed an IP3-specific radioreceptor binding assay to directly measure the IP3 levels before and after NSP4 challenge. NSP4 at a maximally effective dose of 100 nM induced a 3.67 ± 0.52 fold (n = 3; duplicates in each experiment) increase in IP3 production over basal levels (Fig. 6). This response peaked 15 s after agonist addition and declined slowly toward basal levels in 2 min. The kinetics of the NSP4-induced IP3 production was similar to that of neurotensin used in control experiments (data not shown), which has been well documented in the literature (18–20). Basal levels of IP3 for NSP4 and neurotensin assays were similar (1,950 ± 138 cpm, n = 3, in duplicates). Neurotensin (50 nM) stimulated an overall 10.4 ± 0.8-fold increase over basal IP3 levels (n = 3; data not shown). Neurotensin at a maximal dose was therefore more than twice as efficient at stimulating IP3 production than NSP4. The rapid onset of IP3 produced by both agonists correlated well with the kinetics of the [Ca2+]i increase recorded in these cells. These results suggest that NSP4 mobilizes [Ca2+]i by activating PLC-stimulated IP3 production.
Figure 6.
Kinetics of the NSP4-induced stimulation of IP3 levels in HT-29 cells. Plated cells (107 per well) were incubated with 100 nM NSP4 for the indicated times, and their IP3 content was measured by a specific radioreceptor assay. The data were the means ± SEM (n = 3; each sample was analyzed in duplicate).
DISCUSSION
This study is the first demonstration of the Ca2+-mobilizing effect of the rotavirus enterotoxin NSP4 in human intestinal cells. Our data indicate that NSP4 mobilizes [Ca2+]i in HT-29 cells by a receptor-mediated process that involves PLC activation and IP3 production.
NSP4 Induces a Transient [Ca2+]i Rise as Well as a Ca2+ Influx in HT-29 Cells.
The ED50 for NSP4-induced peak [Ca2+]i mobilization was similar to that of neurotensin. Comparing the NSP4 and neurotensin effects in HT-29 cells, the lower levels of cellular IP3 accumulation induced by NSP4 may account for its significantly smaller peak [Ca2+]i rise. Interestingly, prior superfusion with neurotensin significantly delayed the NSP4-induced [Ca2+]i response (13 ± 1 min), whereas NSP4 challenge before neurotensin superfusion had little temporal effect (18 ± 6 s) on the [Ca2+]i mobilization elicited by neurotensin. This indicates that NSP4 mobilizes [Ca2+]i from a subset of the neurotensin-sensitive Ca2+ pools.
NSP4-induced intracellular Ca2+ store release is also accompanied by extracellular Ca2+ influx into the cells. NSP4 at doses as high as 500 nM did not alter the [Na+]i homeostasis, nor did it result in the release of fura-2 from the cells. The [Ca2+]i mobilization elicited by NSP4 therefore reflects both internal Ca2+ store release and ion-specific plasma membrane Ca2+ influx. We predict that the combination of these two processes underlies the gradual rise in [Ca2+]i in rotavirus-infected MA104 cells reported by Michelangeli and coworkers (6, 7).
Ca2+-Dependency and the Pathogenesis of Some Diarrheal Agents.
Rotavirus pathogenesis has also been associated with VP3, VP4, VP7, NSP1, and NSP2 viral proteins. However, their relationship to the diarrheal response remains unknown (reviewed in ref. 41). [Ca2+]i mobilization by receptor-mediated processes have been shown to activate endogenous Ca2+-dependent Cl− secretory pathways in both immature gastrointestinal cells and in epithelial cells found in other tissues (30, 31). Based on our observations, we speculate that NSP4 may induce diarrhea during the earliest stages of viral infection in the small intestine by mobilizing [Ca2+]i within enterocytes to promote and/or evoke Ca2+-dependent fluid secretion across the mucosa.
Rotavirus-infected mouse cells when viewed ultrastructurally have been shown to contain enlarged ER cisternae and vacuoles beneath the apical membrane (42–44). Viral replication in mice is not associated with gross morphological changes within the mucosa. Studies conducted in other animal models have demonstrated that the diarrhea associated with the early stages of rotavirus infection appears to be mediated by enhanced fluid transport before gross ultrastructural changes in the mucosa occur. Neonatal pigs inoculated with porcine rotavirus developed watery diarrhea 8 h after infection, whereas minor ultrastructural changes in jejunum segments were observed at 48 h after infection (45). Ball and coworkers have shown that NSP4 injected intraperitoneally or intraileally into young mice induces diarrhea within 1–4 h after inoculation without significant changes in mucosal morphology (ref. 9 and unpublished data). These studies predict that rotavirus-induced secretory diarrhea is initiated by NSP4 and not by ultrastructural alterations in mucosal integrity.
Altered Ca2+ homeostasis has been associated with diarrhea induced by a variety of other infectious pathogens in the gut. Diarrhea is commonly reported in patients with HIV infection. At the cellular level, the HIV envelope glycoprotein gp120 mobilizes [Ca2+]i from caffeine-sensitive intracellular stores in HT-29 cells by binding to a galactosylceramide-containing molecule (46). It has been speculated that either gp120 associated with HIV viral particles or free gp120, which are known to be present in the intestinal mucosa of infected individuals, may increase [Ca2+]i in enterocytes and thereby promote secretory diarrhea (46). Similarly, the heat-stable enterotoxin B (STb) of Escherichia coli has been suggested to induce Ca2+ influx in HT-29 cells by a G protein-mediated mechanism (47). STb is a byproduct from the enterotoxigenic E. coli which causes travelers diarrhea and the majority of acute infectious diarrhea among children living in the developing world (48). Both these and our studies suggest that [Ca2+]i mobilization by different molecular mechanisms may underlie the diarrhea induced by a variety of viral and bacterial pathogens.
In summary, we have delineated the first steps in the NSP4 signal transduction pathway and provided evidence that NSP4 is responsible for some aspects of rotavirus pathogenesis. Our results suggest the existence of a NSP4 receptor, and we speculate that an endogenous ligand for this receptor exists. Our understanding of the molecular mechanisms of the rotavirus enterotoxin NSP4 effect will help us to search for novel therapies for the disease.
Acknowledgments
This work was supported by National Institutes of Health/Pediatric Gastroenterology Training Grant T32 DK07664, National Institutes of Health/National Research Service Award AI09469 to Y.D., National Institutes of Health Grant DK30144 to M.K.E., and Texas Advanced Technology Program Grant 004949-062 to A.P.M. and M.K.E.
ABBREVIATIONS
- ER
endoplasmic reticulum
- IP3
inositol 1,4,5-trisphosphate
- PLC
phospholipase C
- SBFI/AM
sodium-binding benzofuran isophthalate acetoxymethyl ester
- [Ca2+]i
intracellular calcium concentration
References
- 1.Petrie B L, Estes M K, Graham D Y. J Virol. 1983;46:270–274. doi: 10.1128/jvi.46.1.270-274.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dubois-Dalcq M, Holmes K V, Rentier B. In: Assembly of Enveloped RNA Viruses. Kingsbury D W, editor. New York: Spring; 1984. [Google Scholar]
- 3.Au K-S, Chan W-K, Estes M K. J Virol. 1989;63:4553–4562. doi: 10.1128/jvi.63.11.4553-4562.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meyer J C, Bergmann C C, Bellamy A R. Virology. 1989;171:98–107. doi: 10.1016/0042-6822(89)90515-1. [DOI] [PubMed] [Google Scholar]
- 5.Poruchynsky M S, Maass D R, Atkinson P H. J Cell Biol. 1991;114:651–661. doi: 10.1083/jcb.114.4.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Michelangeli F, Ruiz M-C, del Castillo R, Ludert J E, Liprandi F. Virology. 1991;181:520–527. doi: 10.1016/0042-6822(91)90884-e. [DOI] [PubMed] [Google Scholar]
- 7.Michelangeli F, Liprandi F, Chemello M E, Ciarlet M, Ruitz M-C. J Virol. 1995;69:3838–3847. doi: 10.1128/jvi.69.6.3838-3847.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tian P, Hu Y-F, Schilling W P, Lindsay D A, Eiden J, Estes M K. J Virol. 1994;68:251–257. doi: 10.1128/jvi.68.1.251-257.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ball J M, Tian P, Zeng C Q-Y, Morris A P, Estes M K. Science. 1996;272:101–104. doi: 10.1126/science.272.5258.101. [DOI] [PubMed] [Google Scholar]
- 10.Augeron C, Laboisse C L. Cancer Res. 1984;44:3961–3969. [PubMed] [Google Scholar]
- 11.Darmoul D, Lacasa M, Baricault L, Marguet D, Sapin C, Trotot P, Barbat A, Trugnan G. J Biol Chem. 1992;267:4824–4833. [PubMed] [Google Scholar]
- 12.Tanaka K, Masu M, Nakanishi S. Neuron. 1990;4:847–854. doi: 10.1016/0896-6273(90)90137-5. [DOI] [PubMed] [Google Scholar]
- 13.Turner J T, James-Kracke M R, Camden J M. J Pharmacol Exp Ther. 1990;253:1049–1056. [PubMed] [Google Scholar]
- 14.Evers B M, Ishizuka J, Chung D H, Townsend C M, Jr, Thompson J C. Ann Surg. 1990;216:423–430. doi: 10.1097/00000658-199210000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kopp R, Lambrecht G, Mutschler E, Moser U, Tacke R, Pfeiffer A. Eur J Pharmacol. 1989;172:397–407. doi: 10.1016/0922-4106(89)90021-7. [DOI] [PubMed] [Google Scholar]
- 16.Sreedharan S P, Huang J-X, Cheung M-C, Goetzl E J. Proc Natl Acad Sci USA. 1995;92:2939–2943. doi: 10.1073/pnas.92.7.2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Amar S, Kitabgi P, Vincent J P. FEBS Lett. 1986;201:31–36. doi: 10.1016/0014-5793(86)80565-8. [DOI] [PubMed] [Google Scholar]
- 18.Bozou J C, Rochet N, Magnaldo I, Vincent J P, Kitabgi P. Biochem J. 1989;264:871–878. doi: 10.1042/bj2640871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nitschke R, Leipziger J, Greger R. Pflügers Arch. 1993;423:519–526. doi: 10.1007/BF00374950. [DOI] [PubMed] [Google Scholar]
- 20.Wolff T, Leipziger J, Fischer K-G, Klar B, Nitschke R, Greger R. Pflügers Arch. 1993;424:423–430. doi: 10.1007/BF00374904. [DOI] [PubMed] [Google Scholar]
- 21.Morris A P, Kirk K L, Frizzell R A. Cell Regul. 1990;1:953–963. doi: 10.1091/mbc.1.12.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morris A P, Cunningham S A, Benos D J, Frizzell R A. J Biol Chem. 1992;267:5575–5583. [PubMed] [Google Scholar]
- 23.Superti F, Tinari A, Baldassarri L, Donelli G. Arch Virol. 1991;116:159–173. doi: 10.1007/BF01319239. [DOI] [PubMed] [Google Scholar]
- 24.Mckinney M M, Parkinson A. J Immunol Methods. 1987;96:271–278. doi: 10.1016/0022-1759(87)90324-3. [DOI] [PubMed] [Google Scholar]
- 25.Grynkiewicz G, Poenie M, Tsien R Y. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- 26.Minta A, Tsien R Y. J Biol Chem. 1989;264:19449–19457. [PubMed] [Google Scholar]
- 27.Harootunian A T, Kao J P Y, Eckert B K, Tsien R Y. J Biol Chem. 1989;264:19458–19467. [PubMed] [Google Scholar]
- 28.Borin M, Siffert W. J Biol Chem. 1990;265:19543–19550. [PubMed] [Google Scholar]
- 29.Palmer S, Hughes K T, Lee D Y, Wakelam M J O. Cell Signalling. 1989;1:147–156. doi: 10.1016/0898-6568(89)90004-1. [DOI] [PubMed] [Google Scholar]
- 30.Morris A P, Frizzell R A. Am J Physiol. 1993;264:C968–C976. doi: 10.1152/ajpcell.1993.264.4.C968. [DOI] [PubMed] [Google Scholar]
- 31.Morris A P, Frizzell R A. Am J Physiol. 1993;264:C977–C985. doi: 10.1152/ajpcell.1993.264.4.C977. [DOI] [PubMed] [Google Scholar]
- 32.Putney J W., Jr Cell Calcium. 1986;7:1–12. doi: 10.1016/0143-4160(86)90026-6. [DOI] [PubMed] [Google Scholar]
- 33.Merritt J E, Jacob R, Hallam T J. J Biol Chem. 1989;264:1522–1527. [PubMed] [Google Scholar]
- 34.Mertz L M, Baum B J, Ambudkar I S. J Biol Chem. 1990;265:15010–15014. [PubMed] [Google Scholar]
- 35.Nystedt S, Emilsson K, Larsson A K, Strombeck B, Sundelin J. Eur J Biochem. 1995;232:84–89. doi: 10.1111/j.1432-1033.1995.tb20784.x. [DOI] [PubMed] [Google Scholar]
- 36.Berridge M J, Irvine R F. Nature (London) 1989;341:197–205. doi: 10.1038/341197a0. [DOI] [PubMed] [Google Scholar]
- 37.Bleasdale J E, Thakur N R, Gremban R S, Bundy G L, Fitzpatrick F A, Smith R J, Bunting S. J Pharmacol Exp Ther. 1990;255:756–768. [PubMed] [Google Scholar]
- 38.Smith R J, Sam L M, Justen J M, Bundy G L, Bala G A, Bleasdale J E. J Pharmacol Exp Ther. 1990;253:688–697. [PubMed] [Google Scholar]
- 39.Schacht J. J Neurochem. 1976;27:1119–1124. doi: 10.1111/j.1471-4159.1976.tb00318.x. [DOI] [PubMed] [Google Scholar]
- 40.Carney D H, Scott D L, Gordon E A, LaBelle E F. Cell. 1985;42:479–488. doi: 10.1016/0092-8674(85)90105-9. [DOI] [PubMed] [Google Scholar]
- 41.Burke B, Desselberger U. Virology. 1996;218:299–305. doi: 10.1006/viro.1996.0198. [DOI] [PubMed] [Google Scholar]
- 42.Starkey W G, Collins J, Wallis T S, Clarke G J, Spencer A J, Haddon S J, Osborne M P, Candy D C A, Stephen J. J Gen Virol. 1986;67:2625–2634. doi: 10.1099/0022-1317-67-12-2625. [DOI] [PubMed] [Google Scholar]
- 43.Osborne M P, Haddon S J, Spencer A J, Collins J, Starkey W G, Wallis T S, Clarke G J, Worton K J, Candy D C A, Stephen J. J Pediatr Gastroenterol Nutr. 1988;7:236–248. doi: 10.1097/00005176-198803000-00014. [DOI] [PubMed] [Google Scholar]
- 44.Greenberg H B, Clark H F, Offit P A. Curr Top Microbiol Immunol. 1994;185:255–583. doi: 10.1007/978-3-642-78256-5_9. [DOI] [PubMed] [Google Scholar]
- 45.Vellenga L, Egberts H J A, Wensing T, van Dijk J E, Mouwen J M V M, Breukink H J. Am J Vet Res. 1992;53:1180–1183. [PubMed] [Google Scholar]
- 46.Dayanithi G, Yahi N, Baghdiguian S, Fantini J. Cell Calcium. 1995;18:9–18. doi: 10.1016/0143-4160(95)90041-1. [DOI] [PubMed] [Google Scholar]
- 47.Dreyfus L A, Harville B, Howard D E, Shaban R, Beatty D M, Morris S J. Proc Natl Acad Sci USA. 1993;90:3202–3206. doi: 10.1073/pnas.90.8.3202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gorbach S L, Kean B H, Evans D G, Evans D J, Jr, Bessudo D. N Engl J Med. 1975;292:933–936. doi: 10.1056/NEJM197505012921801. [DOI] [PubMed] [Google Scholar]