Abstract
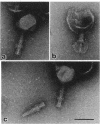
We have isolated and characterized a lytic double-stranded DNA Xanthomonas campestris pv. campestris bacteriophage (XTP1) capable of mediating generalized transduction. The phage transduces chromosomal markers at frequencies of 10(-5) to 10(-6) transductants per PFU. We demonstrated its genetic utility by the isolation and cotransduction of linked transposon insertions to a nonselectable locus, xgl, required for the cleavage of 5-bromo-3-chloro-indoyl-beta-D-galactoside and showed that rif and str alleles in X. campestris are 75% linked. One-step growth experiments showed that the latent and rise periods were each 2 h and the average burst size was 35. The DNA genome is approximately 180 kb, presumably modified in a sequence-specific manner, and may be covalently attached to protein(s). Electron micrographs show the phage particle to have an icosahedral head and contractile tail with tail fibers uniquely attached to a location 40 nm proximal from the end of the tail.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Daniels M. J., Barber C. E., Turner P. C., Sawczyc M. K., Byrde R. J., Fielding A. H. Cloning of genes involved in pathogenicity of Xanthomonas campestris pv. campestris using the broad host range cosmid pLAFR1. EMBO J. 1984 Dec 20;3(13):3323–3328. doi: 10.1002/j.1460-2075.1984.tb02298.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich M., Ehrlich K., Mayo J. A. Unusual properties of the DNA from Xanthomonas phage XP-12 in which 5-methylcytosine completely replaces cytosine. Biochim Biophys Acta. 1975 Jun 16;395(2):109–119. doi: 10.1016/0005-2787(75)90149-5. [DOI] [PubMed] [Google Scholar]
- Figurski D. H., Helinski D. R. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1648–1652. doi: 10.1073/pnas.76.4.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García E., Gómez A., Ronda C., Escarmis C., López R. Pneumococcal bacteriophage Cp-1 contains a protein bound to the 5' termini of its DNA. Virology. 1983 Jul 15;128(1):92–104. doi: 10.1016/0042-6822(83)90321-5. [DOI] [PubMed] [Google Scholar]
- Grimes S., Anderson D. In vitro packaging of bacteriophage phi 29 DNA restriction fragments and the role of the terminal protein gp3. J Mol Biol. 1989 Sep 5;209(1):91–100. doi: 10.1016/0022-2836(89)90172-1. [DOI] [PubMed] [Google Scholar]
- Liu Y. N., Tang J. L., Clarke B. R., Dow J. M., Daniels M. J. A multipurpose broad host range cloning vector and its use to characterise an extracellular protease gene of Xanthomonas campestris pathovar campestris. Mol Gen Genet. 1990 Feb;220(3):433–440. doi: 10.1007/BF00391750. [DOI] [PubMed] [Google Scholar]
- Miller R. V., Pemberton J. M., Richards K. E. F116, D3 and G101: temperate bacteriophages of Pseudomonas aeruginosa. Virology. 1974 Jun;59(2):566–569. doi: 10.1016/0042-6822(74)90466-8. [DOI] [PubMed] [Google Scholar]
- Oeltmann T. N., Heath E. C. Isolation and characterization of a bacteriophage polysaccharide. J Biol Chem. 1975 Nov 25;250(22):8696–8703. [PubMed] [Google Scholar]
- Osbourn A. E., Barber C. E., Daniels M. J. Identification of plant-induced genes of the bacterial pathogen Xanthomonas campestris pathovar campestris using a promoter-probe plasmid. EMBO J. 1987 Jan;6(1):23–28. doi: 10.1002/j.1460-2075.1987.tb04713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rekosh D. M., Russell W. C., Bellet A. J., Robinson A. J. Identification of a protein linked to the ends of adenovirus DNA. Cell. 1977 Jun;11(2):283–295. doi: 10.1016/0092-8674(77)90045-9. [DOI] [PubMed] [Google Scholar]
- Romero A., Lopez R., Lurz R., Garcia P. Temperate bacteriophages of Streptococcus pneumoniae that contain protein covalently linked to the 5' ends of their DNA. J Virol. 1990 Oct;64(10):5149–5155. doi: 10.1128/jvi.64.10.5149-5155.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas M., Davis R. W. Studies on the cleavage of bacteriophage lambda DNA with EcoRI Restriction endonuclease. J Mol Biol. 1975 Jan 25;91(3):315–328. doi: 10.1016/0022-2836(75)90383-6. [DOI] [PubMed] [Google Scholar]
- Trun N. J., Silhavy T. J. Characterization and in vivo cloning of prlC, a suppressor of signal sequence mutations in Escherichia coli K12. Genetics. 1987 Aug;116(4):513–521. doi: 10.1093/genetics/116.4.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Vidaver A. K., Schuster M. L. Characterization of Xanthomonas phaseoli Bacteriophages. J Virol. 1969 Sep;4(3):300–308. doi: 10.1128/jvi.4.3.300-308.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]