Abstract
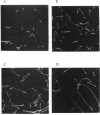
A member of the clpC subfamily of stress response-related Clp ATPases was cloned from Bacillus subtilis. The B. subtilis clpC gene was induced in response to various stresses, including heat shock. Its product was identified as a general stress protein (Gsp12) described previously. A dramatic increase in the amount of clpC mRNA immediately after exposure to multiple stresses suggested regulation on a transcriptional level. Induction by heat shock was independent of the alternative sigma factor SigB, indicating a new mechanism of heat shock induction in B. subtilis. A clpC insertional mutant had an impaired tolerance for heat shock and salt stress. Furthermore, the mutation triggered the formation of elongated cells, a phenomenon particularly pronounced during stress.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnosti D. N., Singer V. L., Chamberlin M. J. Characterization of heat shock in Bacillus subtilis. J Bacteriol. 1986 Dec;168(3):1243–1249. doi: 10.1128/jb.168.3.1243-1249.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson A. K., Haldenwang W. G. The sigma B-dependent promoter of the Bacillus subtilis sigB operon is induced by heat shock. J Bacteriol. 1993 Apr;175(7):1929–1935. doi: 10.1128/jb.175.7.1929-1935.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C. A rapid alkaline extraction method for the isolation of plasmid DNA. Methods Enzymol. 1983;100:243–255. doi: 10.1016/0076-6879(83)00059-2. [DOI] [PubMed] [Google Scholar]
- Boylan S. A., Redfield A. R., Brody M. S., Price C. W. Stress-induced activation of the sigma B transcription factor of Bacillus subtilis. J Bacteriol. 1993 Dec;175(24):7931–7937. doi: 10.1128/jb.175.24.7931-7937.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canceill D., Dervyn E., Huisman O. Proteolysis and modulation of the activity of the cell division inhibitor SulA in Escherichia coli lon mutants. J Bacteriol. 1990 Dec;172(12):7297–7300. doi: 10.1128/jb.172.12.7297-7300.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowds B. C., Murphy P., McConnell D. J., Devine K. M. Relationship among oxidative stress, growth cycle, and sporulation in Bacillus subtilis. J Bacteriol. 1987 Dec;169(12):5771–5775. doi: 10.1128/jb.169.12.5771-5775.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubnau D., Roggiani M. Growth medium-independent genetic competence mutants of Bacillus subtilis. J Bacteriol. 1990 Jul;172(7):4048–4055. doi: 10.1128/jb.172.7.4048-4055.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson J. W., Gross C. A. Identification of the sigma E subunit of Escherichia coli RNA polymerase: a second alternate sigma factor involved in high-temperature gene expression. Genes Dev. 1989 Sep;3(9):1462–1471. doi: 10.1101/gad.3.9.1462. [DOI] [PubMed] [Google Scholar]
- Erickson J. W., Vaughn V., Walter W. A., Neidhardt F. C., Gross C. A. Regulation of the promoters and transcripts of rpoH, the Escherichia coli heat shock regulatory gene. Genes Dev. 1987 Jul;1(5):419–432. doi: 10.1101/gad.1.5.419. [DOI] [PubMed] [Google Scholar]
- Fabisiewicz A., Piechowska M. Changes of sensitivity to heat shock during logarithmic growth of Bacillus subtilis. Acta Biochim Pol. 1988;35(3):207–217. [PubMed] [Google Scholar]
- Ferrari F. A., Nguyen A., Lang D., Hoch J. A. Construction and properties of an integrable plasmid for Bacillus subtilis. J Bacteriol. 1983 Jun;154(3):1513–1515. doi: 10.1128/jb.154.3.1513-1515.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman S., Maurizi M. R. Regulation by proteolysis: energy-dependent proteases and their targets. Microbiol Rev. 1992 Dec;56(4):592–621. doi: 10.1128/mr.56.4.592-621.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman S., Squires C., Pichersky E., Carrington M., Hobbs M., Mattick J. S., Dalrymple B., Kuramitsu H., Shiroza T., Foster T. Conservation of the regulatory subunit for the Clp ATP-dependent protease in prokaryotes and eukaryotes. Proc Natl Acad Sci U S A. 1990 May;87(9):3513–3517. doi: 10.1073/pnas.87.9.3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Hecker M., Heim C., Völker U., Wölfel L. Induction of stress proteins by sodium chloride treatment in Bacillus subtilis. Arch Microbiol. 1988;150(6):564–566. doi: 10.1007/BF00408250. [DOI] [PubMed] [Google Scholar]
- Hoch J. A. Genetic analysis in Bacillus subtilis. Methods Enzymol. 1991;204:305–320. doi: 10.1016/0076-6879(91)04015-g. [DOI] [PubMed] [Google Scholar]
- Igo M., Lampe M., Ray C., Schafer W., Moran C. P., Jr, Losick R. Genetic studies of a secondary RNA polymerase sigma factor in Bacillus subtilis. J Bacteriol. 1987 Aug;169(8):3464–3469. doi: 10.1128/jb.169.8.3464-3469.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M., Wong S. L. Cloning and characterization of the groESL operon from Bacillus subtilis. J Bacteriol. 1992 Jun;174(12):3981–3992. doi: 10.1128/jb.174.12.3981-3992.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindner C., Stülke J., Hecker M. Regulation of xylanolytic enzymes in Bacillus subtilis. Microbiology. 1994 Apr;140(Pt 4):753–757. doi: 10.1099/00221287-140-4-753. [DOI] [PubMed] [Google Scholar]
- Mattick J. S., Anderson B. J., Cox P. T., Dalrymple B. P., Bills M. M., Hobbs M., Egerton J. R. Gene sequences and comparison of the fimbrial subunits representative of Bacteroides nodosus serotypes A to I: class I and class II strains. Mol Microbiol. 1991 Mar;5(3):561–573. doi: 10.1111/j.1365-2958.1991.tb00727.x. [DOI] [PubMed] [Google Scholar]
- Maurizi M. R. Proteases and protein degradation in Escherichia coli. Experientia. 1992 Feb 15;48(2):178–201. doi: 10.1007/BF01923511. [DOI] [PubMed] [Google Scholar]
- Meade H. M., Long S. R., Ruvkun G. B., Brown S. E., Ausubel F. M. Physical and genetic characterization of symbiotic and auxotrophic mutants of Rhizobium meliloti induced by transposon Tn5 mutagenesis. J Bacteriol. 1982 Jan;149(1):114–122. doi: 10.1128/jb.149.1.114-122.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nath I., Laal S. Nucleotide sequence and deduced amino acid sequence of Mycobacterium leprae gene showing homology to bacterial atp operon. Nucleic Acids Res. 1990 Aug 25;18(16):4935–4935. doi: 10.1093/nar/18.16.4935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsell D. A., Sanchez Y., Stitzel J. D., Lindquist S. Hsp104 is a highly conserved protein with two essential nucleotide-binding sites. Nature. 1991 Sep 19;353(6341):270–273. doi: 10.1038/353270a0. [DOI] [PubMed] [Google Scholar]
- Pearce B. J., Yin Y. B., Masure H. R. Genetic identification of exported proteins in Streptococcus pneumoniae. Mol Microbiol. 1993 Sep;9(5):1037–1050. doi: 10.1111/j.1365-2958.1993.tb01233.x. [DOI] [PubMed] [Google Scholar]
- Sadaie Y., Kada T. Bacillus subtilis gene involved in cell division, sporulation, and exoenzyme secretion. J Bacteriol. 1985 Aug;163(2):648–653. doi: 10.1128/jb.163.2.648-653.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez Y., Taulien J., Borkovich K. A., Lindquist S. Hsp104 is required for tolerance to many forms of stress. EMBO J. 1992 Jun;11(6):2357–2364. doi: 10.1002/j.1460-2075.1992.tb05295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt A., Schiesswohl M., Völker U., Hecker M., Schumann W. Cloning, sequencing, mapping, and transcriptional analysis of the groESL operon from Bacillus subtilis. J Bacteriol. 1992 Jun;174(12):3993–3999. doi: 10.1128/jb.174.12.3993-3999.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiroza T., Kuramitsu H. K. Sequence analysis of the Streptococcus mutans fructosyltransferase gene and flanking regions. J Bacteriol. 1988 Feb;170(2):810–816. doi: 10.1128/jb.170.2.810-816.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith I., Paress P., Cabane K., Dubnau E. Genetics and physiology of the rel system of Bacillus subtilis. Mol Gen Genet. 1980;178(2):271–279. doi: 10.1007/BF00270472. [DOI] [PubMed] [Google Scholar]
- Squires C. L., Pedersen S., Ross B. M., Squires C. ClpB is the Escherichia coli heat shock protein F84.1. J Bacteriol. 1991 Jul;173(14):4254–4262. doi: 10.1128/jb.173.14.4254-4262.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squires C., Squires C. L. The Clp proteins: proteolysis regulators or molecular chaperones? J Bacteriol. 1992 Feb;174(4):1081–1085. doi: 10.1128/jb.174.4.1081-1085.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streips U. N., Polio F. W. Heat shock proteins in bacilli. J Bacteriol. 1985 Apr;162(1):434–437. doi: 10.1128/jb.162.1.434-437.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stülke J., Hanschke R., Hecker M. Temporal activation of beta-glucanase synthesis in Bacillus subtilis is mediated by the GTP pool. J Gen Microbiol. 1993 Sep;139(9):2041–2045. doi: 10.1099/00221287-139-9-2041. [DOI] [PubMed] [Google Scholar]
- Todd J. A., Hubbard T. J., Travers A. A., Ellar D. J. Heat-shock proteins during growth and sporulation of Bacillus subtilis. FEBS Lett. 1985 Sep 2;188(2):209–214. doi: 10.1016/0014-5793(85)80373-2. [DOI] [PubMed] [Google Scholar]
- Tybulewicz V. L., Falk G., Walker J. E. Rhodopseudomonas blastica atp operon. Nucleotide sequence and transcription. J Mol Biol. 1984 Oct 25;179(2):185–214. doi: 10.1016/0022-2836(84)90465-0. [DOI] [PubMed] [Google Scholar]
- Völker U., Engelmann S., Maul B., Riethdorf S., Völker A., Schmid R., Mach H., Hecker M. Analysis of the induction of general stress proteins of Bacillus subtilis. Microbiology. 1994 Apr;140(Pt 4):741–752. doi: 10.1099/00221287-140-4-741. [DOI] [PubMed] [Google Scholar]
- Völker U., Mach H., Schmid R., Hecker M. Stress proteins and cross-protection by heat shock and salt stress in Bacillus subtilis. J Gen Microbiol. 1992 Oct;138(10):2125–2135. doi: 10.1099/00221287-138-10-2125. [DOI] [PubMed] [Google Scholar]
- Wada K., Wada Y., Doi H., Ishibashi F., Gojobori T., Ikemura T. Codon usage tabulated from the GenBank genetic sequence data. Nucleic Acids Res. 1991 Apr 25;19 (Suppl):1981–1986. doi: 10.1093/nar/19.suppl.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetzstein M., Völker U., Dedio J., Löbau S., Zuber U., Schiesswohl M., Herget C., Hecker M., Schumann W. Cloning, sequencing, and molecular analysis of the dnaK locus from Bacillus subtilis. J Bacteriol. 1992 May;174(10):3300–3310. doi: 10.1128/jb.174.10.3300-3310.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuber U., Schumann W. CIRCE, a novel heat shock element involved in regulation of heat shock operon dnaK of Bacillus subtilis. J Bacteriol. 1994 Mar;176(5):1359–1363. doi: 10.1128/jb.176.5.1359-1363.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]