Abstract
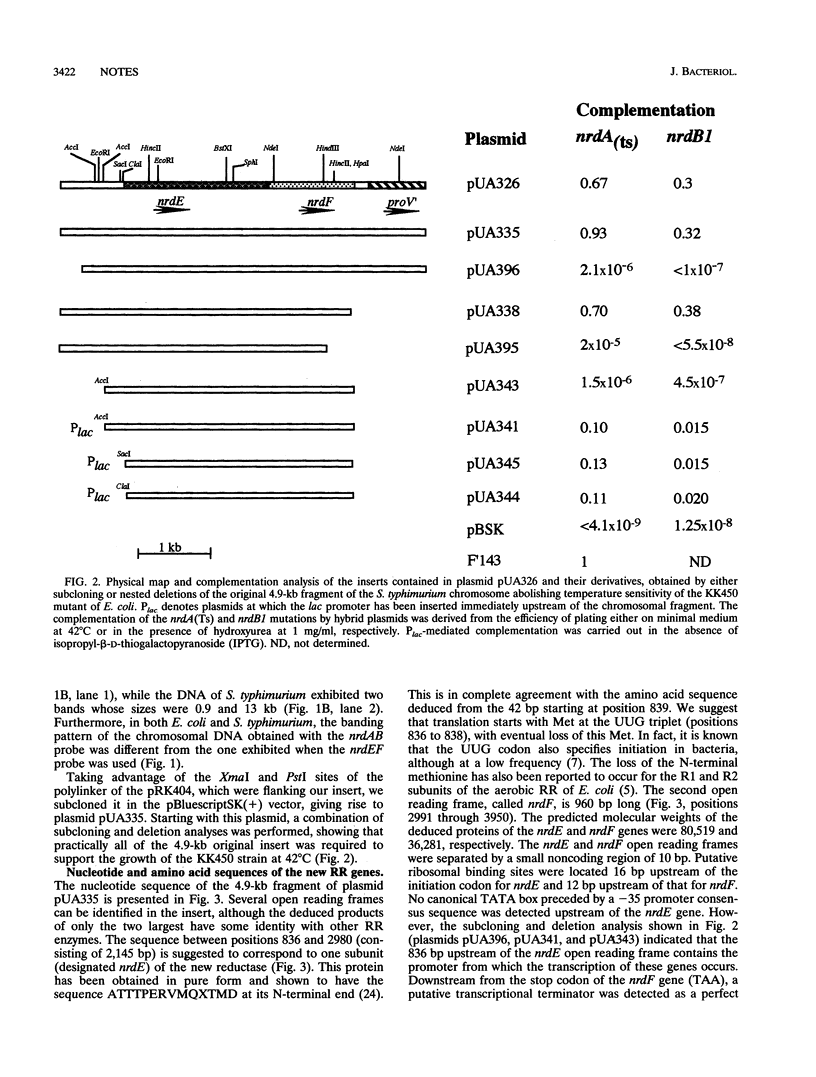
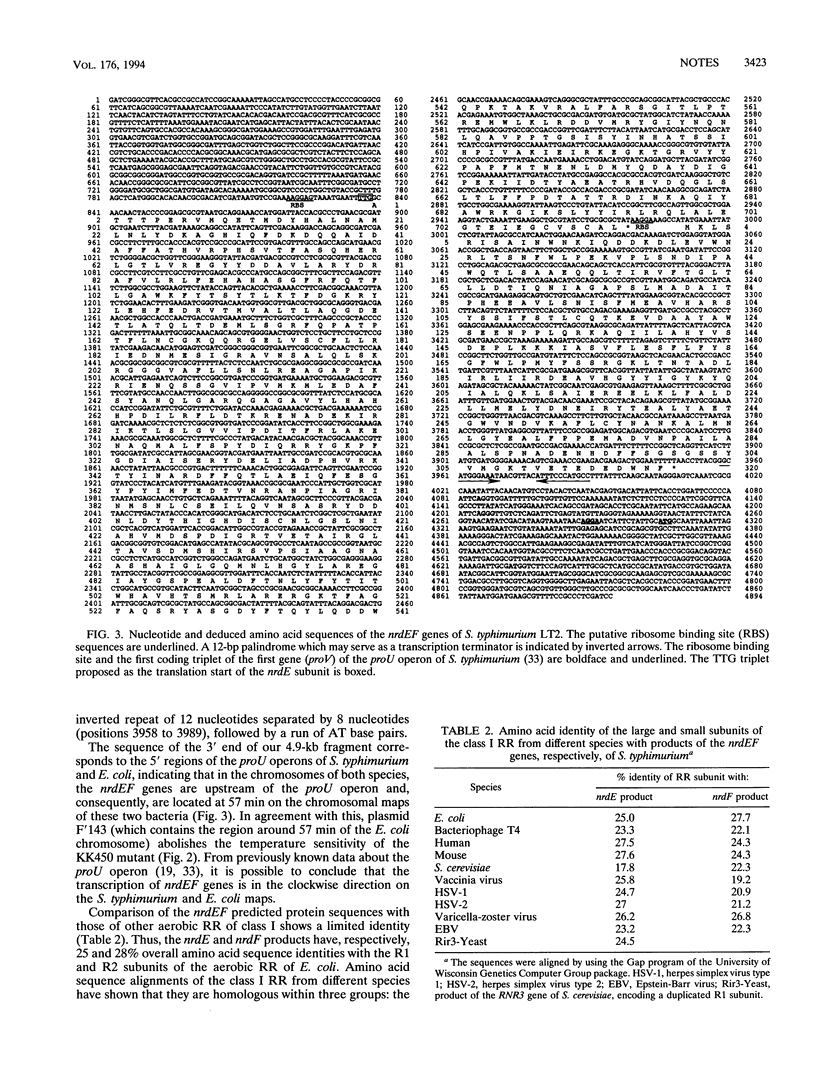
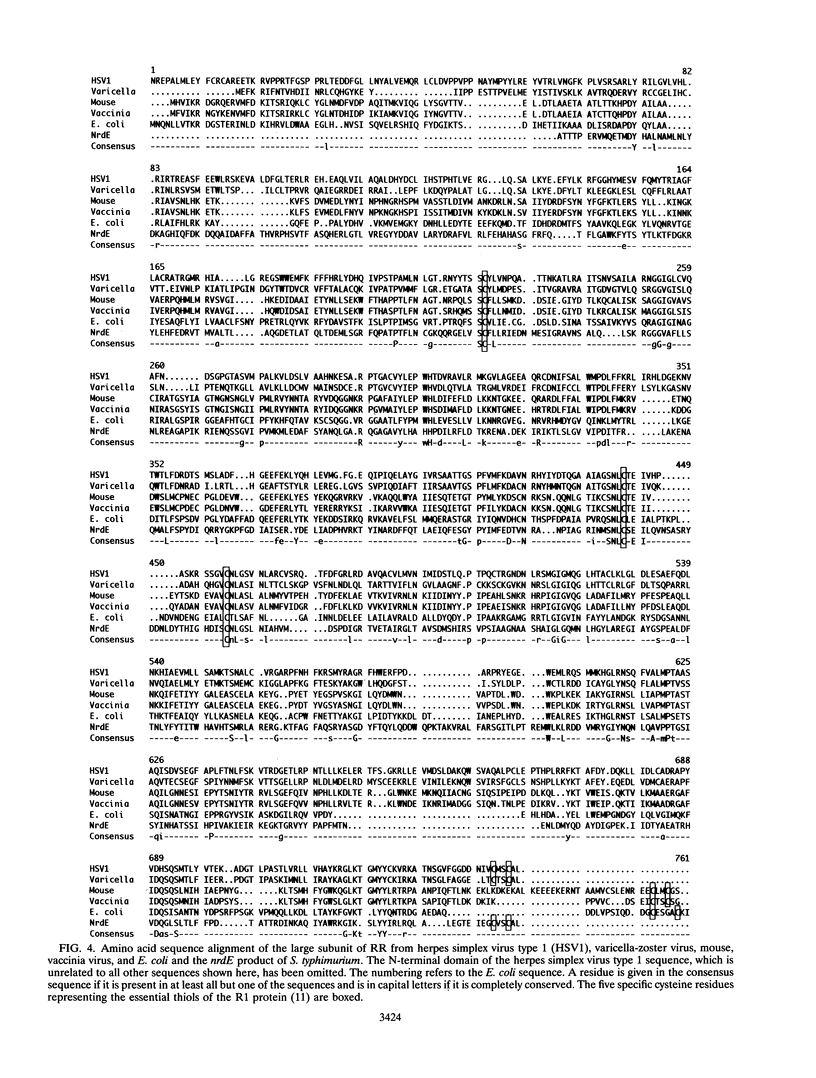
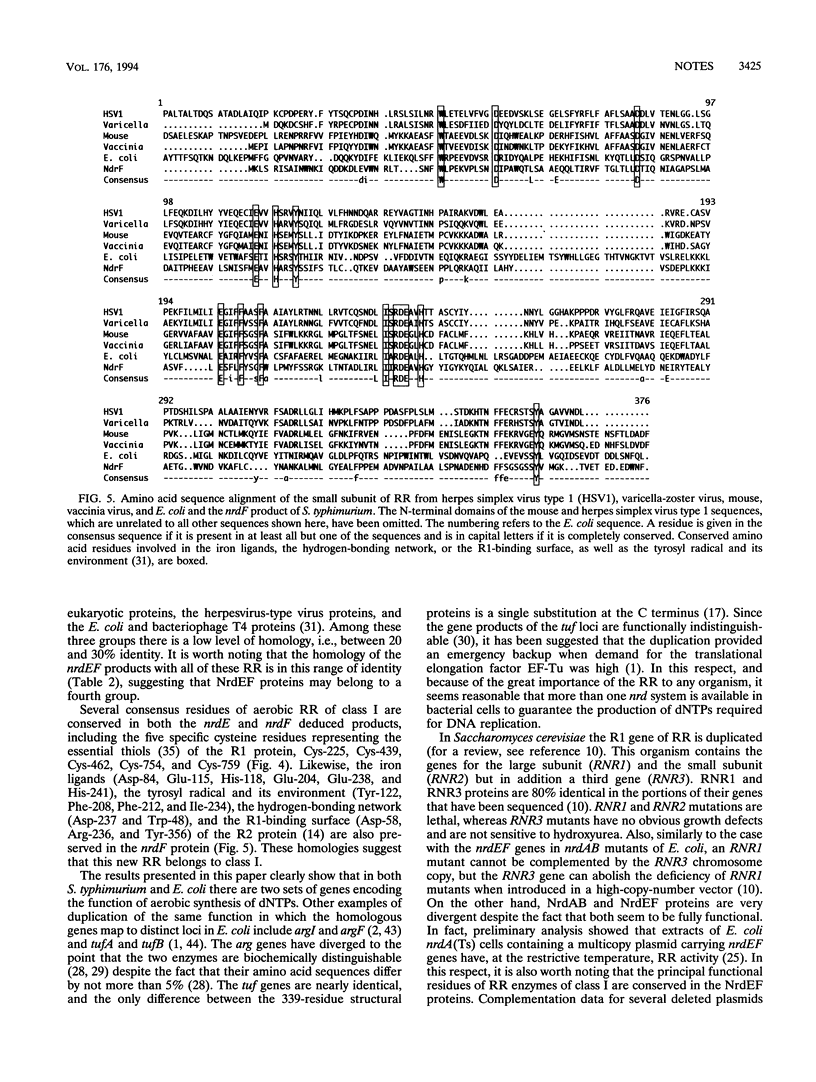
A plasmid library of Salmonella typhimurium was used to complement a temperature-sensitive nrdA mutant of Escherichia coli. Complementation was obtained with two different classes of plasmids, one carrying the E. coli nrdAB-like genes and the second containing an operon encoding a new bacterial ribonucleotide reductase. Plasmids harboring these new reductase genes also enable obligately anaerobic nrdB::Mud1 E. coli mutants to grow in the presence of oxygen. This operon consists of two open reading frames, which have been designated nrdE (2,145 bp) and nrdF (969 bp). The deduced amino acid sequences of the nrdE and nrdF products include the catalytically important residues conserved in ribonucleotide reductase enzymes of class I and show 25 and 28% overall identity with the R1 and R2 protein, respectively, of the aerobic ribonucleoside diphosphate reductase of E. coli. The 3' end of the sequenced 4.9-kb fragment corresponds to the upstream region of the previously published proU operon of both S. typhimurium and E. coli, indicating that the nrdEF genes are at 57 min on the chromosomal maps of these two bacterial species. Analysis of the nrdEF and proU sequences demonstrates that transcription of the nrdEF genes is in the clockwise direction on the S. typhimurium and E. coli maps.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- An G., Friesen J. D. The nucleotide sequence of tufB and four nearby tRNA structural genes of Escherichia coli. Gene. 1980 Dec;12(1-2):33–39. doi: 10.1016/0378-1119(80)90013-x. [DOI] [PubMed] [Google Scholar]
- Bencini D. A., Houghton J. E., Hoover T. A., Foltermann K. F., Wild J. R., O'Donovan G. A. The DNA sequence of argI from Escherichia coli K12. Nucleic Acids Res. 1983 Dec 10;11(23):8509–8518. doi: 10.1093/nar/11.23.8509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakley R. L., Ghambeer R. K., Batterham T. J., Brownson C. Studies with hydrogen isotopes on the mechanism of action of cobamide-dependent ribonucleotide reductase. Biochem Biophys Res Commun. 1966 Aug 12;24(3):418–426. doi: 10.1016/0006-291x(66)90176-8. [DOI] [PubMed] [Google Scholar]
- Carlson J., Fuchs J. A., Messing J. Primary structure of the Escherichia coli ribonucleoside diphosphate reductase operon. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4294–4297. doi: 10.1073/pnas.81.14.4294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casado C., Llagostera M., Barbé J. Expression of nrdA and nrdB genes of Escherichia coli is decreased under anaerobiosis. FEMS Microbiol Lett. 1991 Oct 1;67(2):153–157. doi: 10.1016/0378-1097(91)90346-c. [DOI] [PubMed] [Google Scholar]
- Clark B. F., Marcker K. A. The role of N-formyl-methionyl-sRNA in protein biosynthesis. J Mol Biol. 1966 Jun;17(2):394–406. doi: 10.1016/s0022-2836(66)80150-x. [DOI] [PubMed] [Google Scholar]
- Ditta G., Schmidhauser T., Yakobson E., Lu P., Liang X. W., Finlay D. R., Guiney D., Helinski D. R. Plasmids related to the broad host range vector, pRK290, useful for gene cloning and for monitoring gene expression. Plasmid. 1985 Mar;13(2):149–153. doi: 10.1016/0147-619x(85)90068-x. [DOI] [PubMed] [Google Scholar]
- Dower W. J., Miller J. F., Ragsdale C. W. High efficiency transformation of E. coli by high voltage electroporation. Nucleic Acids Res. 1988 Jul 11;16(13):6127–6145. doi: 10.1093/nar/16.13.6127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elledge S. J., Zhou Z., Allen J. B. Ribonucleotide reductase: regulation, regulation, regulation. Trends Biochem Sci. 1992 Mar;17(3):119–123. doi: 10.1016/0968-0004(92)90249-9. [DOI] [PubMed] [Google Scholar]
- Filpula D., Fuchs J. A. Regulation of ribonucleoside diphosphate reductase synthesis in Escherichia coli: increased enzyme synthesis as a result of inhibition of deoxyribonucleic acid synthesis. J Bacteriol. 1977 Apr;130(1):107–113. doi: 10.1128/jb.130.1.107-113.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontecave M., Eliasson R., Reichard P. Oxygen-sensitive ribonucleoside triphosphate reductase is present in anaerobic Escherichia coli. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2147–2151. doi: 10.1073/pnas.86.7.2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontecave M., Nordlund P., Eklund H., Reichard P. The redox centers of ribonucleotide reductase of Escherichia coli. Adv Enzymol Relat Areas Mol Biol. 1992;65:147–183. doi: 10.1002/9780470123119.ch4. [DOI] [PubMed] [Google Scholar]
- Fuchs J. A. Coordinate control of the synthesis of ribonucleoside diphosphate reductase components in Escherichia coli. J Bacteriol. 1977 May;130(2):957–959. doi: 10.1128/jb.130.2.957-959.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs J. A., Karlström H. O. A mutant of Escherichia coli defective in ribonucleosidediphosphate reductase. 2. Characterization of the enzymatic defect. Eur J Biochem. 1973 Feb 1;32(3):457–462. [PubMed] [Google Scholar]
- Furano A. V. The elongation factor Tu coded by the tufA gene of Escherichia coli K-12 is almost identical to that coded by the tufB gene. J Biol Chem. 1977 Mar 25;252(6):2154–2157. [PubMed] [Google Scholar]
- Gibert I., Calero S., Barbé J. Measurement of in vivo expression of nrdA and nrdB genes of Escherichia coli by using lacZ gene fusions. Mol Gen Genet. 1990 Feb;220(3):400–408. doi: 10.1007/BF00391745. [DOI] [PubMed] [Google Scholar]
- Gowrishankar J. Nucleotide sequence of the osmoregulatory proU operon of Escherichia coli. J Bacteriol. 1989 Apr;171(4):1923–1931. doi: 10.1128/jb.171.4.1923-1931.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hantke K. Characterization of an iron sensitive Mud1 mutant in E. coli lacking the ribonucleotide reductase subunit B2. Arch Microbiol. 1988;149(4):344–349. doi: 10.1007/BF00411654. [DOI] [PubMed] [Google Scholar]
- Higgins C. F., Dorman C. J., Stirling D. A., Waddell L., Booth I. R., May G., Bremer E. A physiological role for DNA supercoiling in the osmotic regulation of gene expression in S. typhimurium and E. coli. Cell. 1988 Feb 26;52(4):569–584. doi: 10.1016/0092-8674(88)90470-9. [DOI] [PubMed] [Google Scholar]
- Kunz B. A. Genetic effects of deoxyribonucleotide pool imbalances. Environ Mutagen. 1982;4(6):695–725. doi: 10.1002/em.2860040609. [DOI] [PubMed] [Google Scholar]
- Larsson A., Sjöberg B. M. Identification of the stable free radical tyrosine residue in ribonucleotide reductase. EMBO J. 1986 Aug;5(8):2037–2040. doi: 10.1002/j.1460-2075.1986.tb04461.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legrain C., Halleux P., Stalon V., Glansdorff N. The dual genetic control of ornithine carbamolytransferase in Escherichia coli. A case of bacterial hybrid enzymes. Eur J Biochem. 1972 May;27(1):93–102. doi: 10.1111/j.1432-1033.1972.tb01814.x. [DOI] [PubMed] [Google Scholar]
- Legrain C., Stalon V., Glansdorff N. Escherichia coli ornithine carbamolytransferase isoenzymes: evolutionary significance and the isolation of lambdaargF and lambdaargI transducing bacteriophages. J Bacteriol. 1976 Oct;128(1):35–38. doi: 10.1128/jb.128.1.35-38.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller D. L. A comparison of the activities of the products of the two genes for elongation factor Tu. Mol Gen Genet. 1978 Feb 7;159(1):57–62. doi: 10.1007/BF00401748. [DOI] [PubMed] [Google Scholar]
- Nordlund P., Eklund H. Structure and function of the Escherichia coli ribonucleotide reductase protein R2. J Mol Biol. 1993 Jul 5;232(1):123–164. doi: 10.1006/jmbi.1993.1374. [DOI] [PubMed] [Google Scholar]
- Ochman H., Wilson A. C. Evolution in bacteria: evidence for a universal substitution rate in cellular genomes. J Mol Evol. 1987;26(1-2):74–86. doi: 10.1007/BF02111283. [DOI] [PubMed] [Google Scholar]
- Overdier D. G., Olson E. R., Erickson B. D., Ederer M. M., Csonka L. N. Nucleotide sequence of the transcriptional control region of the osmotically regulated proU operon of Salmonella typhimurium and identification of the 5' endpoint of the proU mRNA. J Bacteriol. 1989 Sep;171(9):4694–4706. doi: 10.1128/jb.171.9.4694-4706.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platz A., Sjöberg B. M. Construction and characterization of hybrid plasmids containing the Escherichia coli nrd region. J Bacteriol. 1980 Aug;143(2):561–568. doi: 10.1128/jb.143.2.561-568.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichard P. From RNA to DNA, why so many ribonucleotide reductases? Science. 1993 Jun 18;260(5115):1773–1777. doi: 10.1126/science.8511586. [DOI] [PubMed] [Google Scholar]
- Reichard P. The anaerobic ribonucleotide reductase from Escherichia coli. J Biol Chem. 1993 Apr 25;268(12):8383–8386. [PubMed] [Google Scholar]
- Riley M. Functions of the gene products of Escherichia coli. Microbiol Rev. 1993 Dec;57(4):862–952. doi: 10.1128/mr.57.4.862-952.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X., Harder J., Krook M., Jörnvall H., Sjöberg B. M., Reichard P. A possible glycine radical in anaerobic ribonucleotide reductase from Escherichia coli: nucleotide sequence of the cloned nrdD gene. Proc Natl Acad Sci U S A. 1993 Jan 15;90(2):577–581. doi: 10.1073/pnas.90.2.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taschner P. E., Verest J. G., Woldringh C. L. Genetic and morphological characterization of ftsB and nrdB mutants of Escherichia coli. J Bacteriol. 1987 Jan;169(1):19–25. doi: 10.1128/jb.169.1.19-25.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuggle C. K., Fuchs J. A. Regulation of the operon encoding ribonucleotide reductase in Escherichia coli: evidence for both positive and negative control. EMBO J. 1986 May;5(5):1077–1085. doi: 10.1002/j.1460-2075.1986.tb04325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuggle C. K., Fuchs J. A. Regulation of the operon encoding ribonucleotide reductase: role of the negative sites in nrd repression. J Bacteriol. 1990 Apr;172(4):1711–1718. doi: 10.1128/jb.172.4.1711-1718.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Vliet F., Cunin R., Jacobs A., Piette J., Gigot D., Lauwereys M., Piérard A., Glansdorff N. Evolutionary divergence of genes for ornithine and aspartate carbamoyl-transferases--complete sequence and mode of regulation of the Escherichia coli argF gene; comparison of argF with argI and pyrB. Nucleic Acids Res. 1984 Aug 10;12(15):6277–6289. doi: 10.1093/nar/12.15.6277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota T., Sugisaki H., Takanami M., Kaziro Y. The nucleotide sequence of the cloned tufA gene of Escherichia coli. Gene. 1980 Dec;12(1-2):25–31. doi: 10.1016/0378-1119(80)90012-8. [DOI] [PubMed] [Google Scholar]