Abstract
Acute promyelocytic leukemia (APL) is associated with the t(15;17) translocation, which generates a PML/RARα fusion protein between PML, a growth suppressor localized on nuclear matrix-associated bodies, and RARα, a nuclear receptor for retinoic acid (RA). PML/RARα was proposed to block myeloid differentiation through inhibition of nuclear receptor response, as does a dominant negative RARα mutant. In addition, in APL cells, PML/RARα displaces PML and other nuclear body (NB) antigens onto nuclear microspeckles, likely resulting in the loss of PML and/or NB functions. RA leads to clinical remissions through induction of terminal differentiation, for which the respective contributions of RARα (or PML/RARα) activation, PML/RARα degradation, and restoration of NB antigens localization are poorly determined. Arsenic trioxide also leads to remissions in APL patients, presumably through induction of apoptosis. We demonstrate that in non-APL cells, arsenic recruits the nucleoplasmic form of several NB antigens onto NB, but induces the degradation of PML only, identifying a powerful tool to approach NB function. In APL cells, arsenic targets PML and PML/RARα onto NB and induces their degradation. Thus, RA and arsenic target RARα and PML, respectively, but both induce the degradation of the PML/RARα fusion protein, which should contribute to their therapeutic effects. The difference in the cellular events triggered by these two agents likely stems from RA-induced transcriptional activation and arsenic effects on NB proteins.
Keywords: therapy, retinoic acid, protein traffic, nuclear matrix, retinoic acid receptor α
Acute promyelocytic leukemia (APL) is associated with a specific t(15;17) translocation that generates a fusion protein between PML, a zinc finger protein that contains a coiled-coil domain, and a nuclear receptor for retinoic acid, RARα (1). As a dominant negative RARα mutant was shown to inhibit myeloid cell differentiation (2), interference of the fusion protein with nuclear receptor function was proposed to account for the differentiation block observed in this disease. Indeed, transduction of PML/RARα into U937 cells inhibits transforming growth factor type β- and vitamin D3-induced monocytic differentiation (3). The PML coiled-coil and, at least in part, the RARα DNA-binding domain appear to be required for inhibition of differentiation (4).
That two members of the PML family (T18/TIF1 and RFP) are also involved in oncogenic fusions suggests a contribution of the PML moiety of the chimeric PML/RARα protein to transformation (5). PML is localized on discrete subnuclear structures of unknown function previously identified by electron microscopy [PML nuclear bodies (NBs), also known as PML oncogenic domains; refs. 6–9]. These structures (which belong to the nuclear matrix; ref. 10) were previously identified using autoantibodies from patients with primary biliary cirrhosis (11) that recognize a proposed transcription factor, Sp100 (12, 13). The three NB antigens identified to date, Sp100, PML, and NDP52, are all target genes of interferons (IFNs; refs. 14–17). Infections by herpes- or adenoviruses delocalize NB antigens, in particular PML (18–21). Overexpression of PML sharply reduces the growth of some cell lines, abolishes focus formation by cooperating oncogenes on primary cells, and reverses transformation by the neu oncogene (22–24). Altogether, these observations have suggested that PML NBs play a role in growth control by as yet uncharacterized mechanisms. In APL, PML/RARα displaces not only PML, but also the other known NB antigens to other ill-defined nuclear sites, presumably through the formation of PML–PML/RARα heterodimers (5–9). In that sense, the fusion protein exerts a dominant negative activity on PML localization, which could contribute to deregulated cell growth.
APL is a unique model system in cancer biology, because retinoic acid (RA), the ligand for RARα, can induce terminal differentiation of the leukemic cells and induce transient clinical remissions, providing the first example of differentiation therapy (1). APL is also unique in the existence of a treatment directly targeted at the causative genetic lesion. RA was shown to induce the degradation of the fusion protein (25), which could account for the previously described RA-triggered restoration in NB antigen localization (6–9). The respective contribution of RA-induced PML/RARα catabolism versus transcriptional activation of RARα (or PML/RARα) to induction of differentiation remains unknown.
Recently, arsenic trioxide (As2O3), present with cadmium and mercury in numerous Chinese traditional medicines (26), was identified as a very potent antileukemic agent (27–29). As2O3 appears to induce prolonged remissions even in RA-resistant APL cases (27). Although some arsenic accumulates in the body, no obvious toxicity was observed at the doses used. Ex vivo studies on the APL cell line NB4 demonstrate that As2O3 down-regulates bcl-2 expression and induces apoptosis in the absence of apparent differentiation (27). Whether arsenic also induces apoptosis during in vivo therapy is unclear, as some differentiation may occur in NB4 cells treated with lower doses (27). Arsenic also induced modifications of PML/RARα localization, which, however, were also observed in spontaneously apoptotic cells, raising the question of their significance (27). Arsenic did not affect the survival of non-APL cell lines, but it decreased the number of PML bodies (27).
We demonstrate that in non-APL cells, As2O3 induces the targeting of the nucleoplasmic fraction of PML onto the matrix-bound NB, where PML is degraded. In APL cells, PML and PML/RARα are both relocalized and degraded. Thus, RA and arsenic, which target RAR and PML, respectively, both induce the degradation of the fusion protein. That these two drugs induce either differentiation or apoptosis stresses the importance of their other effects on the RARα and PML gene products and could suggest a role of PML in induction of apoptosis.‖
MATERIALS AND METHODS
Construction and Maintenance of Cell Lines.
NB4 cells were grown in RPMI medium supplemented with 10% fetal calf serum, sodium bicarbonate, glutamine, penicillin, and streptomycin. Chinese hamster ovary (CHO) cells were maintained in DMEM medium with the same additives. Arsenic trioxide (As2O3; Sigma) was kept as a 10−4 M stock solution and added to a 10−6 M final concentration. A CHO clone stably expressing a PML isoform (30) was constructed by cotransfecting pSG5-PML together with a hygromycin resistance expression vector (DSP-hygro). A similar procedure was used to express various PML mutants (F.Q., unpublished work). The PML/RARα cDNA (30) was cloned into the IRES-containing vector pCIN (kindly provided by S. Rees, Glaxo) and transfected into CHO–PML cells. A pool of hygromycin- and G418-resistant cells, 95% of which expressed various levels of PML/RARα, was analyzed.
Immunofluorescence Analysis.
Confocal microscopy was performed as previously described (7). PML was revealed by rabbit polyclonal antibodies (6); Sp100 was revealed with a mouse polyclonal serum (18); and RARα, RXRα, and NDP52 were revealed by monoclonal antibodies kindly provided by P. Chambon (Institut de Génétique et de Biologie Moléculaire et Cellulaire, Strasbourg, France), B. Allegreto (Ligand Pharmaceuticals, La Jolla, CA), and G. Maul (Wistar Institute, Philadelphia), respectively. Identical results were obtained with at least two different fixations.
Western Blotting and Immunoprecipitation.
Unless otherwise indicated, cells were directly lysed in Laemmli sample buffer, and 30 μg of proteins was analyzed by SDS/PAGE and Western blotting using rabbit polyclonal antibodies [anti-PML, anti-RARα (RPαF) and anti-RXRα (445) (both kindly provided by P. Chambon), and anti-Sp100]. Antibody complexes were detected by chemoluminescence.
For immunoprecipitation, cells were labeled overnight with [35S]methionine/cysteine (final concentration 300 μCi/ml; 1 Ci = 37 GBq) and lysed in RIPA buffer (50 mM Tris, pH 7.5/200 mM NaCl/1% Triton X-100/0.5% deoxycholate/0.1% SDS/1 mM EDTA), the supernatant (which contains the cytoplasm and most of the nucleoplasm) yielding the RIPA fraction. The nuclear remnants were washed four times in RIPA buffer, boiled in 2% SDS/1 mM EDTA for 10 min to dissociate the remaining nuclear components, and diluted in SDS-less RIPA to reach a final SDS concentration of 0.1%. Immunoprecipitation was performed either with an anti-PML rabbit polyclonal or with a mixture of five anti-PML monoclonal antibodies (M. C. Guillemin, personal communication) yielding identical results. Immune complexes were precipitated with protein G-Sepharose (Pharmacia), washed twice in RIPA buffer containing 1 M NaCl, and analyzed by SDS/PAGE. The gel was fixed, soaked in Amplify (Amersham), dried, and exposed. For the pulse–chase, cells were labeled with 1 mCi/ml [35S]methionine/cysteine for 2 h before the chase with excess unlabeled methionine.
Electron Microscopy.
Glutaraldehyde fixation was performed as described (7), followed by dehydration in graded series of ethanol. Embedding was performed in Epon and thin sections were double-stained with uranyl acetate and lead citrate.
RESULTS
Effects of Arsenic on NB Antigen Localization in APL Cells.
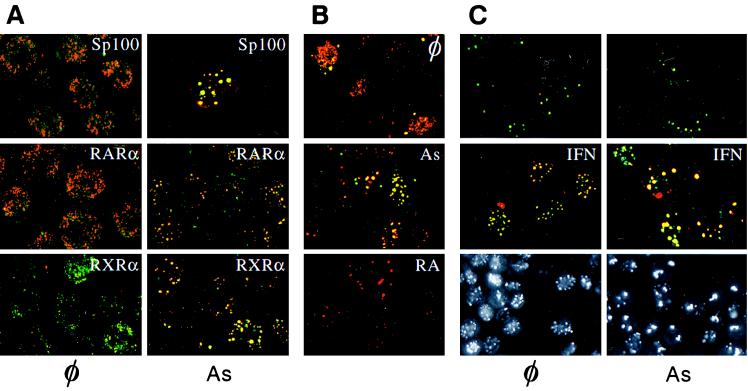
Preliminary evidence had shown that an overnight As2O3 exposure lead to a decrease in PML staining and accumulation of PML fluorescence as large cytoplasmic dots in NB4 cells (27). As these images are also observed in spontaneously apoptotic NB4 cells (ref. 27 and data not shown), we investigated the earlier effects of arsenic (at the therapeutic concentration of 10−6 M) on the localization of the NB antigens [PML, Sp100 (12, 13), and NDP52 (17)] as well as PML/RARα. As2O3 profoundly affected the immunofluorescence pattern obtained with an anti-PML antisera that detects both PML and PML/RARα. A progressive shift was observed from the specific microspeckled NB4 pattern to the normal PML pattern seen in non-APL cells. The first changes were apparent 1 h posttreatment (data not shown) and seemed complete after 6 h (Fig. 1A). Other NB antigens, Sp100 and NDP52, similarly returned onto larger dots (Fig. 1A and data not shown). Longer arsenic treatments (24 h) led to an extinction of PML staining or accumulation of large cytoplasmic dots. Arsenic therefore induces the reaggregation of NB antigens as does RA, but arsenic is a much faster effector than RA. During RA-induced PML relocalization, PML/RARα fluorescence (detected with an anti-RARα) disappeared (7, 8). RXRα became undetectable independently of PML/RARα, as it is transcriptionally down-regulated by RA (31, 32). In contrast, in the presence of arsenic PML/RARα and RXRα accompanied PML onto NBs (Fig. 1A). Identical results were found in three samples of APL blasts in primary culture (data not shown).
Figure 1.
(A) Confocal laser microscopy analysis of NB4 leukemic cells double-labeled with anti-PML antibodies [revealed by fluorescein isothiocyanate (FITC)] and anti-Sp100 (Top), anti-RARα (Middle), and anti-RXRα (Bottom) antibodies (revealed with Texas Red). Cells were treated (Right) or not (Left) with 10−6 M arsenic trioxide for 5 h. (B) Differential effects of RA and As2O3 (10−6 M, 24 h) on PML/RARα localization in CHO PML–PML/RARα cells. Double-staining with anti-PML (Texas Red) and anti-RARα (FITC) antibodies. (C Top and Middle) Arsenic recruits PML and Sp100 onto NB in HeLa cells. Double anti-PML (FITC)/anti-Sp100 (Texas Red) staining. Cells were primed (or not) with 500 units/ml IFNα for 12 h and treated (As) or untreated (⊘) with 10−6 M As2O3 for 7 h. (C Bottom) Immunofluorescence analysis of CHO PML cells. φ, untreated; As, arsenic-treated for 12 h. Note that arsenic induces the disappearance of the diffuse nuclear staining.
To confirm the differential effect of RA and arsenic on PML/RARα localization, we constructed a CHO cell line stably overexpressing PML and PML/RARα, where both proteins colocalized in APL-like microspeckles in most cells. After As2O3 treatment, both PML and PML/RARα were found in NB (Fig. 1B). In contrast, upon RA treatment, PML became associated to NBs and anti-RARα fluorescence disappeared, consistent with our previous observations in NB4 cells. Thus, these two drugs similarly affect the localization of PML, Sp100, and NDP52, but they have distinct effects on PML/RARα and RXRα localization.
Induction of PML/RARα Protein Degradation by Arsenic.
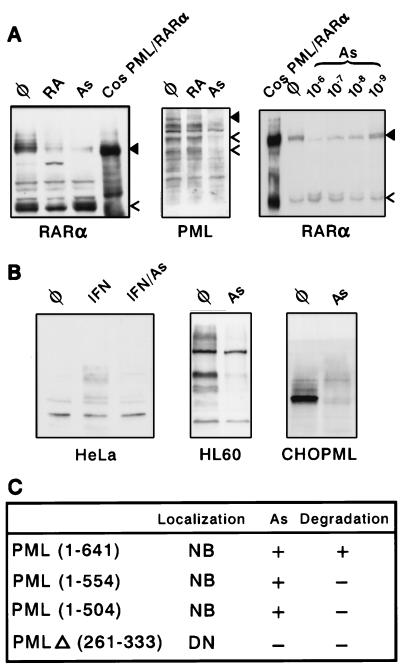
In some reports, RA was shown to induce PML/RARα destruction (25), although this was not consistently observed (7, 32). Indeed, in NB4 cells, RA led to the degradation of PML/RARα on a Western blot, had minor effects on RARα, and no effect on PML expression (Fig. 2A). Interestingly, a specific protein species is consistently observed after RA treatment. As the antibody used recognizes RARα C terminus, the putative cleavage site should be in the PML part of the fusion. This abundant protein may thus retain some transcriptional activation properties. Strikingly, an 8-h As2O3 exposure led to a 10- to 20-fold reduction in PML/RARα expression. Degradation was also induced by 10−7 M As2O3 (or other trivalent arsenicals) and 10−5 M sodium arsenate (Fig. 2A and data not shown). Wild-type RARα was not degraded, but a higher molecular weight form [previously shown to be a phosphorylated receptor (33)] increased in a dose-dependent manner (Fig. 2A). The endogenous PML proteins also disappeared, suggesting that PML is the target of As2O3 action (Fig. 2A). Identical results were obtained in two samples of primary APL blasts similarly exposed to arsenic ex vivo (data not shown). Despite the fact that they were relocalized onto NBs, the levels of Sp100 and RXRα proteins were unaltered (data not shown). It should be noted that the decrease in PML/RARα expression (Fig. 2A) is accompanied by an increase in immunofluorescence (Fig. 1A) attributable to the recruitment of the fusion protein onto NB during degradation. As the remaining protein is concentrated on a much smaller nuclear domain, it becomes brighter than previously. Due to the low level of PML/RARα expression, CHO PML–PML/RARα cells were not suitable for Western blot analysis.
Figure 2.
Western blot analysis of As2O3 effects in APL or non-APL cells (A Left and Center) NB4 cells were treated for 12 h with arsenic (As), RA, or untreated (φ) and probed with the antibodies indicated. (Right) NB4 cells were arsenic-treated for 8 h. The solid arrowhead points to PML/RARα, and the open arrowhead points to RARα (Left and Right) or PML (Center). Positive controls are extracts from transiently transfected COS cells. (B) Endogenous PML proteins from HeLa (Left) or HL60 cells (Center) are down-regulated by an overnight arsenic treatment, as is a stably overexpressed PML isoform in CHO cells (Right). (C) Arsenic sensitivity of PML mutants. NB, NB-associated; DN, diffuse nuclear. As (+) denotes PML recruitment onto NBs.
In Non-APL Cells, Arsenic Recruits PML Proteins onto NBs and Induces Their Degradation.
We then examined the effect of As2O3 on NB protein localization and levels of expression in non-APL cells. For PML, Sp100 and NDP52, the NB-associated fluorescence was enhanced after 7 h of As2O3 treatment in HeLa cells, particularly after IFN induction of the expression of these three proteins (refs. 14–17; Fig. 1C and data not shown). Longer As2O3 exposures lead to a decrease in PML fluorescence and NB number. On a Western blot of HeLa or HL60 cell extracts, endogenous PML proteins (which are present as multiple isoforms generated by alternative splicing (16, 34, 35) were completely degraded after a 12-h As2O3 exposure, consistent with the results seen in NB4 cells (Fig. 2B). Here again, an initial recruitment of PML leads to brighter speckles, but is followed by degradation. Sp100 was also recruited onto NBs (Fig. 1C), but not degraded (data not shown), providing a first point of distinction among the different NB antigens.
In CHO or NIH 3T3 cells stably overexpressing a specific PML isoform, arsenic led to the disappearance of the previously described diffuse nucleoplasmic form of PML (ref. 23; Fig. 1C), and the appearance of fewer, larger, often deformed bodies that remained strongly labeled (see below). Again, in both cell lines, despite the bright speckles, PML expression was drastically reduced on Western blots (Fig. 2B and data not shown). The arsenic dose–response of PML degradation closely resembles that of PML/RARα (data not shown). Stable expression of PML mutants in CHO cells showed that As2O3 enhanced the NB targeting of all NB-associated mutants (C-terminal deletions), but was unable to target onto NBs those with a diffuse nucleoplasmic localization (mutants with a deletion in the coiled-coil; Fig. 2C). Only the full-size protein was degraded in response to arsenic, suggesting the existence of at least a degradation signal in the C terminus of PML (Fig. 2C). Thus, the structural requirement for arsenic-induced PML catabolism is more stringent than for NB targeting.
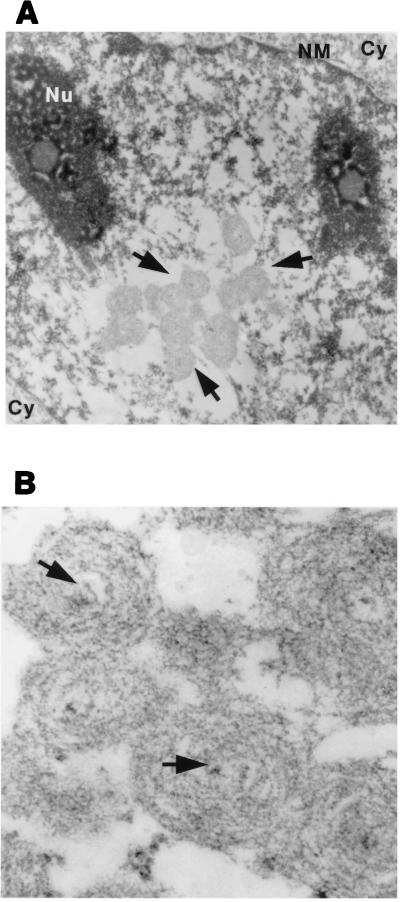
The deformed bodies seen in PML-overexpressing cells treated with arsenic were studied by electron microscopy (Fig. 3 A and B). The PML-labeled structures are large spherical masses that coalesce to form grape-like (Fig. 3A) or rod-like (data not shown) aggregates that may reach the size of a nucleolus. At a higher magnification, the PML-labeled structures have an electron-dense nucleus (arrow in Fig. 3B), which is surrounded by concentric layers of fibrillous material. The sudden arsenic-induced targeting of such a large amount of PML onto these bodies (which are never observed in normal cells) likely accounts for the altered ratio between their electron-dense nucleus and the large PML-containing core.
Figure 3.
Electron microscopic analysis of arsenic treated-cells. (A) Low magnification. Nu, nucleolus; NM, nuclear membrane; Cy, cytoplasm. Note the grape-like structure (arrow), which is highly labeled by PML antibodies (data not shown). (B) High magnification of the PML-containing structures. Note the size ratio between the electron-dense core (arrow) and the multilayered, PML-containing shell (see ref. 7 for comparison).
Arsenic Shifts PML from the Nucleoplasm onto the Nuclear Matrix.
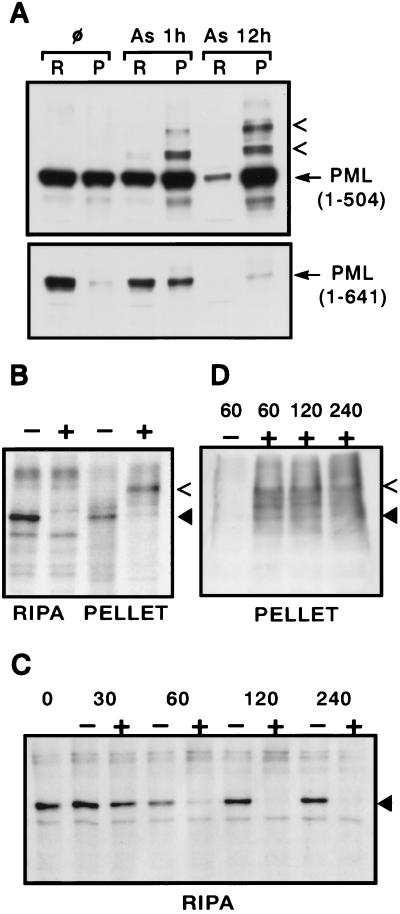
To biochemically detect the NB targeting of PML, PML contents of the RIPA fraction (the cytoplasm and most of the nucleoplasm) and of the nuclear remnants (the nuclear matrix and some chromatin components) were compared before and after arsenic exposure. By Western blot, a clear shift was observed as early as 1 h after exposure (Fig. 4A). After 6 h, high-molecular weight protein adducts are found, almost exclusively in the pellet fraction (data not shown). After 12 h, little PML remained in the pellet fraction for wild-type PML. However, for PML(1–504), the protein was completely recovered, almost exclusively in the pellet fraction, with some high-molecular weight protein adducts (Fig. 4A), consistent with our previous observations in unfractionated cells (Fig. 2C). Similar results were obtained by immunoprecipitating [35S]methionine-labeled cell extracts: PML completely disappeared from the RIPA fraction after a 1-h arsenic treatment and was partially recovered from the nuclear remnants, predominantly as higher-molecular weight proteins (Fig. 4B and data not shown). A pulse–chase experiment gave similar results, establishing the arsenic-induced transfer from the nucleoplasm to the matrix (Fig. 4 C and D). Note that labeled PML proteins are immunoprecipitated from the matrix fraction, only after an overnight labeling, but not after a 2-h pulse, consistent with the idea that PML also traffics from the nucleoplasm to the matrix in the absence of arsenic.
Figure 4.
Arsenic induces matrix transfer of PML before its degradation. (A) Western blot analysis of extracts from arsenic-treated CHO PML (1–641) or PML (1–504) cells. The amounts of protein in the two fractions (R, RIPA; P, pellet) analyzed correspond to the same number of cells. The arrowheads point to modified PML proteins. (B) Immunoprecipitation analysis of RIPA or pellet fractions from CHO PML cells exposed (+) or not (−) to 10−6 M As2O3 for 1 h. PML is indicated by a filled triangle, a high-molecular weight protein by an arrowhead. (C and D) Pulse–chase analysis of arsenic induced nuclear transfer. The fraction analyzed [Pellet (D) and RIPA (C)], the time after chase (in min) and the presence (+) or absence (−) of arsenic are indicated.
DISCUSSION
In this report, we demonstrate that arsenic addresses the nucleoplasmic fraction of the PML protein onto NBs and induces its degradation, identifying an important tool to approach NB function. In APL, arsenic induces the degradation of the PML/RARα fusion protein, pointing to an unexpected convergence between the effects of RA and arsenic. However, that these two agents induce apoptosis and differentiation, respectively, suggests that their action on the normal PML and RARα proteins also significantly contributes to their biological effects.
We demonstrate that PML partitions between the nucleoplasm and the nuclear matrix-associated PML bodies and shuttles from the first to the second. The diffuse nucleoplasmic fraction was previously described (23, 36), but its quantitative importance (at least in some situations) was clearly underestimated. In a previous study, PML was shown to be exclusively a matrix protein (10). The ratio between the PML contents of the nuclear matrix and the nucleoplasm may reflect the saturation of the targeting process and hence vary with respect to the level of PML expression, the cell type, or the growth conditions. Note in this respect that, in HeLa cells, the effect of arsenic is particularly clear only after IFN induction of PML expression. Whether PML shuttles back from NBs to the nucleoplasm (for example at the G1/S transition) or alternatively are NB dead ends for the PML protein is not known. As2O3 did not affect the localization (nor the abundance) of PML (or PML/RARα) in transiently transfected COS or CHO cells. This observation may suggest that As2O3 does not act directly on the protein, but on a rate-limiting posttranslational modification implicated in the NB/matrix association of the protein. The latter might be phosphorylation, as arsenicals are well known to inhibit phosphatases or kinases, although As2O3 inhibits a larger range of enzymes through binding sulfhydryl residues in their active sites. As2O3 induced the phosphorylation of RARα (Fig. 2A), but did not drastically modify the phosphorylation state of PML, as shown by immunoprecipitation of 32P-labeled proteins (data not shown). Nevertheless, phosphorylation of a few residues in PML may control its intranuclear localization. The latter was shown to vary during the cell cycle (23), suggesting that PML might be a substrate for cell cycle-regulated kinases. As the diffuse nuclear form could be the biologically active one and NBs could be storage/sequestration sites, arsenic control of PML and NB protein localization should provide a powerful tool for the dissection of NB function. For example, arsenic recruits Sp100 onto NBs but fails to degrade this protein. Should Sp100 exert some functions out of NB (such as transcriptional activation (12, 13)), arsenic-induced Sp100 sequestration would then abolish it.
Arsenic trioxide induces the degradation of endogenous or stably overexpressed PML proteins. The rapid transfer from the nucleoplasm to NBs and the slower disappearance of PML from the matrix compartment suggests that degradation takes place on NB (Fig. 4 A and C). Analysis of mutant PML proteins uncouples NB targeting from protein degradation, suggesting that the mechanisms involved are distinct (Figs. 2C and 3). Clearly, the 86 C-terminal aa are required for degradation (Fig. 2C), but prior NB-association seems to be required. Whether other regions are implicated in arsenic-induced catabolism awaits further analysis. The formation of covalently modified PML derivatives in the nucleoplasm before degradation is strongly suggestive for the formation of protein adducts, possibly via ubiquitination. Interestingly, a PML-binding protein was recently identified as an ubiquitin-like protein, consistent with this hypothesis (37).
The mechanisms of PML/RARα degradation by these two drugs are likely different. With RA, but not arsenic, a specific cleavage product of the fusion is generated that may retain some transcriptional activation properties. RA-induced PML/RARα degradation most likely results from the direct binding of RA to the fusion protein (38). Consequently, PML/RARα dominant negative effect on PML, Sp100, and NDP52 localization vanishes and these proteins (as well as a very small fraction, if any, of PML/RARα) return onto NBs (7, 8). In arsenic-treated NB4 cells, the PML/RARα fusion is targeted from a nonmatrix compartment onto NBs and then degraded (Figs. 1A and 2A and data not shown). Although arsenic recruits the PML moiety of the PML/RARα fusion [PML (1–554)] toward NBs, it fails to induce its catabolism. Arsenic might directly induce the catabolism of the fusion using a degradation signal in its RARα moiety. Alternatively, arsenic could indirectly target the fusion protein through the PML component of a PML–PML/RARα heterodimer. The toxicity of PML/RARα (3) and its low level of expression in NB4 cells render difficult a detailed analysis of the mechanisms involved in its degradation in response to As2O3.
It is striking that two unrelated APL therapies both lead to the degradation of the fusion protein. While RA targets its RARα moiety and arsenic its PML moiety, the convergence between those two pathways is unexpected and opens the case for rational antileukemic pharmacology. That a 10−7 M arsenic concentration suffices to induce PML/RARα degradation could suggest that lower doses might be used in the clinical setting. Arsenic does not appear to modify RA response, at least in transient transfections with a RA-responsive promoter (ref. 30; data not shown). PML/RARα degradation most likely contributes to the therapeutic response to these two drugs. However, that both RA and arsenic degrade the fusion and yet differently affect NB4 cells suggests that these drugs have other functions. By relieving the differentiation block, PML/RARα degradation would be expected to restore myeloid maturation. However, this is not observed during ex vivo treatment of NB4 cells, probably as they go into apoptosis before differentiation can occur (27). Whether differentiation occurs during arsenic therapy of APL patients is unknown. PML suppresses focus formation by cooperating oncogenes, and PML/RARα antagonizes apoptosis induced by growth factor deprivation (3, 22). Thus, transcriptional activation of RARs (and PML/RARα) by RA could induce differentiation, whereas arsenic targeting of PML (and NB-proteins) onto nuclear dots could lead to apoptosis.
Acknowledgments
We warmly thank M. Schmid (Institut National de la Santé et de la Recherche Médicale Unité 93, Hôpital St. Louis, Paris) for the confocal analysis, E. Puvion (Centre National de la Recherche Scientifique Unité Propre de Recherche 272, Villejuif, France) for the electron microscopy, and M.L. Giron for advice on immunoprecipitations. We gratefully acknowledge the generous gifts of antibodies from Prof. P. Chambon, Dr. M. P. Gaub, Dr. Maul, and Dr. E. Allegreto. We also thank D. Louvard, F. Sigaux, M. H. Stern, T. Soussi, and all members of the H.d.T. laboratory for critical reading of the manuscript. This work was supported by grants from Association pour la Recherche sur le Cancer, Ligue Contre le Cancer (Nationale and Comité de Paris), Fondation St. Louis, and Fondation de France. J.Z., Z.Y.W., and Z.C. were supported by Programme de Recherches Avancées and the National Natural Science Foundation of China (NNSFC).
ABBREVIATIONS
- APL
acute promyelocytic leukemia
- NB
nuclear body
- IFN
interferon
- RA
retinoic acid
- RARα
a nuclear RA receptor
Note Added in Proof
Since the completion of this work, further studies have demonstrated that low-dose As2O3 induces a partial differentiation of ND4 or APL patients’ cells (39).
Footnotes
This work was presented at the Nobel Symposium on “Functional Organization of the Eukaryotic Cell Nucleus,” held September 3–6, 1996, at Saltsjobaden, Sweden.
References
- 1.Warrell R, de Thé H, Wang Z, Degos L. New Engl J Med. 1993;329:177–189. doi: 10.1056/NEJM199307153290307. [DOI] [PubMed] [Google Scholar]
- 2.Tsai S, Bartelmez S, Heyman R, Damm K, Evans R, Collins S. Genes Dev. 1993;6:2258–2269. doi: 10.1101/gad.6.12a.2258. [DOI] [PubMed] [Google Scholar]
- 3.Grignani F, Ferrucci P, Testa U, Talamo G, Fagioli M, Alcalay M, Mencarelli A, Grignani F, Peschle C, Nicoletti I, Pelicci P. Cell. 1993;74:423–431. doi: 10.1016/0092-8674(93)80044-f. [DOI] [PubMed] [Google Scholar]
- 4.Grignani F, Testa U, Rogaia D, Ferrucci P, Samoggia P, Pinto A, Aldinucci D, Gelmetti V, Fagioli M, Alcalay M, Seeler J, Grignani F, Nicoletti I, Peschle C, Pelicci P. EMBO J. 1996;15:4949–4958. [PMC free article] [PubMed] [Google Scholar]
- 5.Kastner P, Perez A, Lutz Y, Rochette-Egly C, Gaub M P, Durand B, Lanotte M, Berger R, Chambon P. EMBO J. 1992;11:629–642. doi: 10.1002/j.1460-2075.1992.tb05095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Daniel M, Koken M, Romagné O, Barbey S, Bazarbachi A, Stadler M, Guillemin M, Degos L, Chomienne C, de Thé H. Blood. 1993;82:1858–1867. [PubMed] [Google Scholar]
- 7.Koken M H M, Puvion-Dutilleul F, Guillemin M C, Viron A, Linares-Cruz G, Stuurman N, de Jong L, Szostecki C, Calvo F, Chomienne C, Degos L, Puvion E, de Thé H. EMBO J. 1994;13:1073–1083. doi: 10.1002/j.1460-2075.1994.tb06356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dyck J A, Maul G G, Miller W H, Chen J D, Kakizuka A, Evans R M. Cell. 1994;76:333–343. doi: 10.1016/0092-8674(94)90340-9. [DOI] [PubMed] [Google Scholar]
- 9.Weis K, Rambaud S, Lavau C, Jansen J, Carvalho T, Carmo-Fonseca M, Lamond A, Dejean A. Cell. 1994;76:345–356. doi: 10.1016/0092-8674(94)90341-7. [DOI] [PubMed] [Google Scholar]
- 10.Stuurman N, de Graaf A, Floore A, Josso A, Humbel B, de Jong L, van Driel R. J Cell Sci. 1992;101:773–84. doi: 10.1242/jcs.101.4.773. [DOI] [PubMed] [Google Scholar]
- 11.Ascoli C A, Maul G G. J Cell Biol. 1991;112:785–795. doi: 10.1083/jcb.112.5.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Szostecki C, Guldner H, Netter H, Will H. J Immunol. 1990;145:4338–4347. [PubMed] [Google Scholar]
- 13.Xie K, Lambie E, Snyder M. Mol Cell Biol. 1993;13:6170–6179. doi: 10.1128/mcb.13.10.6170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guldner H, Szostecki C, Grotzinger T, Will H. J Immunol. 1992;149:4067–4073. [PubMed] [Google Scholar]
- 15.Stadler M, Chelbi-Alix M K, Koken M H M, Venturini L, Lee C, Saïb A, Quignon F, Pelicano L, Guillemin M-C, Schindler C, de Thé H. Oncogene. 1995;11:2565–2573. [PubMed] [Google Scholar]
- 16.Chelbi-Alix M K, Pelicano L, Quignon F, Koken M H M, Venturini L, Stadler M, Pavlovic J, Degos L, de Thé H. Leukemia. 1995;9:2027–2033. [PubMed] [Google Scholar]
- 17.Korioth F, Gieffers C, Maul G G, Frey J. J Cell Biol. 1995;130:1–13. doi: 10.1083/jcb.130.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Puvion-Dutilleul F, Chelbi-Alix M K, Koken M, Quignon F, Puvion E, de Thé H. Exp Cell Res. 1995;218:9–16. doi: 10.1006/excr.1995.1125. [DOI] [PubMed] [Google Scholar]
- 19.Carvalho T, Seeler J-S, Öhman K, Jordan P, Pettersson U, Akusjärvi G, Carmo-Fonseca M, Dejean A. J Cell Biol. 1995;131:45–56. doi: 10.1083/jcb.131.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Doucas V, Ishov A, Romo A, Juguilon H, Weitzman M, Evans R, Maul G. Genes Dev. 1996;10:196–207. doi: 10.1101/gad.10.2.196. [DOI] [PubMed] [Google Scholar]
- 21.Maul G G, Everett R D. J Gen Virol. 1994;75:1223–1233. doi: 10.1099/0022-1317-75-6-1223. [DOI] [PubMed] [Google Scholar]
- 22.Mu Z M, Chin K V, Liu J H, Lozano G, Chang K S. Mol Cell Biol. 1994;14:6858–6867. doi: 10.1128/mcb.14.10.6858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koken M H M, Linares-Cruz G, Quignon F, Viron A, Chelbi-Alix M K, Sobczak-Thépot J, Juhlin L, Degos L, Calvo F, de Thé H. Oncogene. 1995;10:1315–1324. [PubMed] [Google Scholar]
- 24.Liu J-H, Mu Z-M, Chang K-S. J Exp Med. 1995;181:1965–1973. doi: 10.1084/jem.181.6.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yoshida H, Kitamura K, Tanaka K, Omura S, Miyazaki T, Hachiya T, Ohno R, Naoe T. Cancer Res. 1996;56:2945–2948. [PubMed] [Google Scholar]
- 26.Espinoza E, Mann M, Bleasdell B. New Engl J Med. 1995;333:803–804. doi: 10.1056/NEJM199509213331217. [DOI] [PubMed] [Google Scholar]
- 27.Chen G-Q, Zhu J, Shi X-G, Ni J-H, Zhong H-J, Si G-Y, Jin X-L, et al. Blood. 1996;88:1052–1061. [PubMed] [Google Scholar]
- 28.Zhang P, Wang S Y, Hu L H, Shi F D, Qiu F D, Hong R J, Han X Y, Yang H F, Song Y Z, Liu Y P, Zhou J, Jin Z J. Chin J Hematol. 1996;17:58–60. [Google Scholar]
- 29.Sun H D, Ma L, Hu H X, Zhang T D. Chin J Integr Chin West Med. 1992;12:170–171. [Google Scholar]
- 30.de Thé H, Lavau C, Marchio A, Chomienne C, Degos L, Dejean A. Cell. 1991;66:675–684. doi: 10.1016/0092-8674(91)90113-d. [DOI] [PubMed] [Google Scholar]
- 31.Perez A, Kastner P, Sethi S, Lutz Y, Reibel C, Chambon P. EMBO J. 1993;12:3171–3182. doi: 10.1002/j.1460-2075.1993.tb05986.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Duprez E, Lillehaug J R, Gaub M P, Lanotte M. Oncogene. 1996;12:2443–2450. [PubMed] [Google Scholar]
- 33.Gaub M P, Rochette-Egly C, Lutz Y, Ali S, Matthes H, Scheuer I, Chambon P. Exp Cell Res. 1992;201:335–346. doi: 10.1016/0014-4827(92)90282-d. [DOI] [PubMed] [Google Scholar]
- 34.Fagioli M, Alcalay M, Pandolfi P P, Venturini L, Mencarelli A, Simeone A, Acampora D, Grignani F, Pelicci P G. Oncogene. 1992;7:1083–1091. [PubMed] [Google Scholar]
- 35.Goddard A D, Borrow J, Freemont P S, Solomon E. Science. 1991;254:1371–1374. doi: 10.1126/science.1720570. [DOI] [PubMed] [Google Scholar]
- 36.Terris B, Baldin V, Dubois S, Degott C, Flejou J F, Henin D, Dejean A. Cancer Res. 1995;55:1590–1597. [PubMed] [Google Scholar]
- 37.Boddy M N, Howe K, Etkin L D, Solomon E, Freemont P S. Oncogene. 1996;13:971–982. [PubMed] [Google Scholar]
- 38.Nervi C, Poindexter E C, Grignani F, Pandolfi P P, Lo Coco F, Avvisati G, Pelicci P G, Jetten A M. Cancer Res. 1992;52:3687–92. [PubMed] [Google Scholar]
- 39.Chen, G.-Q., Shi, X.-G., Tang, W., Xiong, S.-M., Zhu, J., Cai, X., Han, Z.-G., Ni, J.-H., Shi, G.-Y., Jia, P.-M., Liu, M.-M., He, K.-L., Niu, C., Ma, J., Zhang, P., Zhang, T.-D., Paul, P., Waxman, S., Wang, Z.-Y., Chen, S. & Chen, Z. (1997) Blood, in press. [PubMed]