Abstract
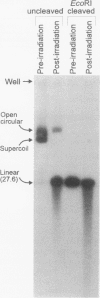
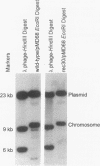
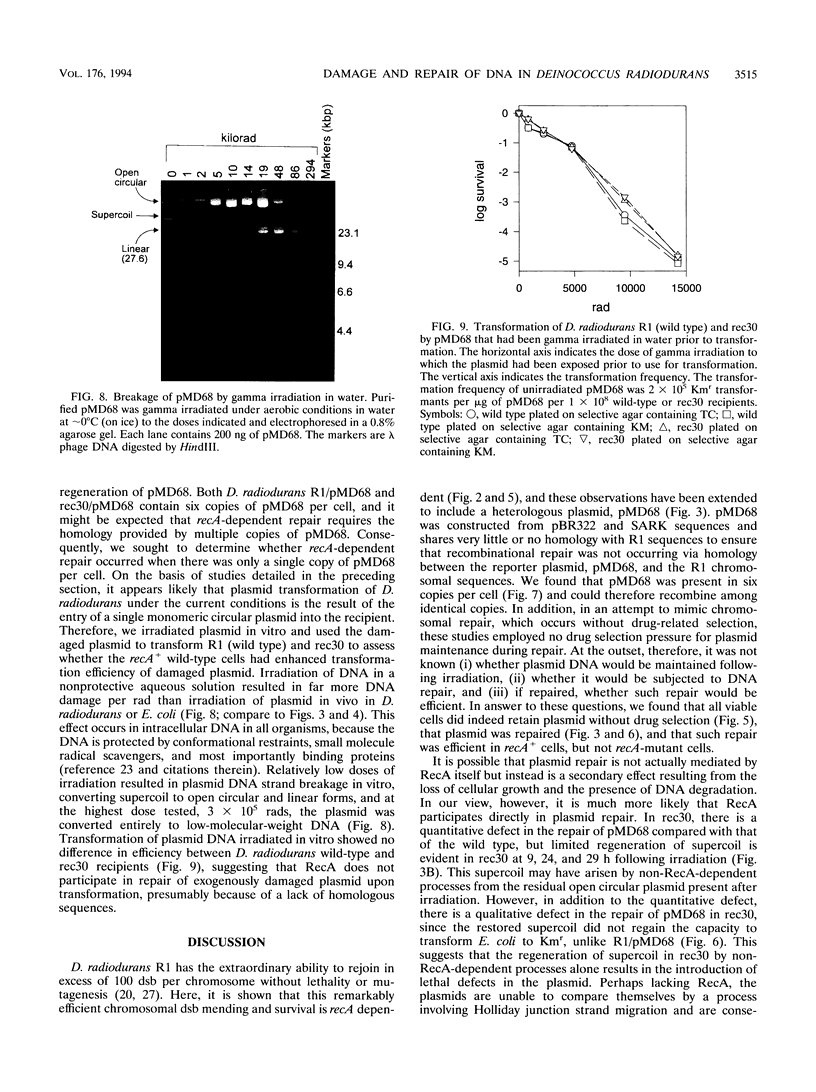
Deinococcus radiodurans R1 and other members of this genus share extraordinary resistance to the lethal and mutagenic effects of ionizing radiation. We have recently identified a RecA homolog in strain R1 and have shown that mutation of the corresponding gene causes marked radiosensitivity. We show here that following high-level exposure to gamma irradiation (1.75 megarads, the dose required to yield 37% of CFU for plateau-phase wild-type R1), the wild-type strain repairs > 150 double-strand breaks per chromosome, whereas a recA-defective mutant (rec30) repairs very few or none. A heterologous Escherichia coli-D. radiodurans shuttle plasmid (pMD68) was constructed and found to be retained in surviving D. radiodurans R1 and rec30 following any radiation exposure up to the highest dose tested, 3 megarads. Plasmid repair was monitored in vivo following irradiation with 1.75 megarads in both R1/pMD68 and rec30/pMD68. Immediately after irradiation, plasmids from both strains contained numerous breaks and failed to transform E. coli. While irradiation with 1.75 megarads was lethal to rec30 cultures, a small amount of supercoiled plasmid was regenerated, but it lacked the ability to transform E. coli. In contrast, wild-type cultures showed a cell division arrest of about 10 h, followed by exponential growth. Supercoiled plasmid was regenerated at normal levels, and it readily transformed E. coli. These studies show that D. radiodurans retains a heterologous plasmid following irradiation and repairs it with the same high efficiency as its chromosomal DNA, while the repair defect in rec30 prevents repair of the plasmid. Taken together, the results of this study suggest that plasmid DNA damaged in vivo in D. radiodurans is repaired by recA-dependent mechanisms similar to those employed in the repair of chromosomal DNA.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonura T., Bruce A. K. The repair of single-strand breaks in a radiosensitive mutant of Micrococcus radiodurans. Radiat Res. 1974 Feb;57(2):260–275. [PubMed] [Google Scholar]
- Burrell A. D., Feldschreiber P., Dean C. J. DNA-membrane association and the repair of double breaks in x-irradiated Micrococcus radiodurans. Biochim Biophys Acta. 1971 Sep 30;247(1):38–53. doi: 10.1016/0005-2787(71)90805-7. [DOI] [PubMed] [Google Scholar]
- Chou F. I., Tan S. T. Manganese(II) induces cell division and increases in superoxide dismutase and catalase activities in an aging deinococcal culture. J Bacteriol. 1990 Apr;172(4):2029–2035. doi: 10.1128/jb.172.4.2029-2035.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean C. J., Feldschreiber P., Lett J. T. Repair of x-ray damage to the deoxyribonucleic acid in Micrococcus radiodurans. Nature. 1966 Jan 1;209(5018):49–52. doi: 10.1038/209049a0. [DOI] [PubMed] [Google Scholar]
- Dianov G. L., Saparbaev M. K., Mazin A. V., Salganik R. I. The chemical mutagen dimethyl sulphate induces homologous recombination of plasmid DNA by increasing the binding of RecA protein to duplex DNA. Mutat Res. 1991 Jul;249(1):189–193. doi: 10.1016/0027-5107(91)90145-e. [DOI] [PubMed] [Google Scholar]
- Fishman-Lobell J., Rudin N., Haber J. E. Two alternative pathways of double-strand break repair that are kinetically separable and independently modulated. Mol Cell Biol. 1992 Mar;12(3):1292–1303. doi: 10.1128/mcb.12.3.1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimsley J. K., Masters C. I., Clark E. P., Minton K. W. Analysis by pulsed-field gel electrophoresis of DNA double-strand breakage and repair in Deinococcus radiodurans and a radiosensitive mutant. Int J Radiat Biol. 1991 Oct;60(4):613–626. doi: 10.1080/09553009114552441. [DOI] [PubMed] [Google Scholar]
- Gutman P. D., Carroll J. D., Masters C. I., Minton K. W. Sequencing, targeted mutagenesis and expression of a recA gene required for the extreme radioresistance of Deinococcus radiodurans. Gene. 1994 Apr 8;141(1):31–37. doi: 10.1016/0378-1119(94)90124-4. [DOI] [PubMed] [Google Scholar]
- Hall S. D., Kane M. F., Kolodner R. D. Identification and characterization of the Escherichia coli RecT protein, a protein encoded by the recE region that promotes renaturation of homologous single-stranded DNA. J Bacteriol. 1993 Jan;175(1):277–287. doi: 10.1128/jb.175.1.277-287.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han E. K., Saffran W. A. Differential repair and recombination of psoralen damaged plasmid DNA in Saccharomyces cerevisiae. Mol Gen Genet. 1992 Dec;236(1):8–16. doi: 10.1007/BF00279637. [DOI] [PubMed] [Google Scholar]
- Hansen M. T. Multiplicity of genome equivalents in the radiation-resistant bacterium Micrococcus radiodurans. J Bacteriol. 1978 Apr;134(1):71–75. doi: 10.1128/jb.134.1.71-75.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harsojo, Kitayama S., Matsuyama A. Genome multiplicity and radiation resistance in Micrococcus radiodurans. J Biochem. 1981 Sep;90(3):877–880. doi: 10.1093/oxfordjournals.jbchem.a133544. [DOI] [PubMed] [Google Scholar]
- Keller L. C., Maxcy R. B. Effect of physiological age on radiation resistance of some bacteria that are highly radiation resistant. Appl Environ Microbiol. 1984 May;47(5):915–918. doi: 10.1128/aem.47.5.915-918.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennon E., Minton K. W. Gene fusions with lacZ by duplication insertion in the radioresistant bacterium Deinococcus radiodurans. J Bacteriol. 1990 Jun;172(6):2955–2961. doi: 10.1128/jb.172.6.2955-2961.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy D. D., Groopman J. D., Lim S. E., Seidman M. M., Kraemer K. H. Sequence specificity of aflatoxin B1-induced mutations in a plasmid replicated in xeroderma pigmentosum and DNA repair proficient human cells. Cancer Res. 1992 Oct 15;52(20):5668–5673. [PubMed] [Google Scholar]
- Ljungman M., Nyberg S., Nygren J., Eriksson M., Ahnström G. DNA-bound proteins contribute much more than soluble intracellular compounds to the intrinsic protection against radiation-induced DNA strand breaks in human cells. Radiat Res. 1991 Aug;127(2):171–176. [PubMed] [Google Scholar]
- Masters C. I., Minton K. W. Promoter probe and shuttle plasmids for Deinococcus radiodurans. Plasmid. 1992 Nov;28(3):258–261. doi: 10.1016/0147-619x(92)90057-h. [DOI] [PubMed] [Google Scholar]
- Masters C. I., Smith M. D., Gutman P. D., Minton K. W. Heterozygosity and instability of amplified chromosomal insertions in the radioresistant bacterium Deinococcus radiodurans. J Bacteriol. 1991 Oct;173(19):6110–6117. doi: 10.1128/jb.173.19.6110-6117.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moseley B. E., Copland H. J. Isolation and properties of a recombination-deficient mutant of Micrococcus radiodurans. J Bacteriol. 1975 Feb;121(2):422–428. doi: 10.1128/jb.121.2.422-428.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudgett J. S., Buckholt M., Taylor W. D. Ultraviolet light-induced plasmid-chromosome recombination in Escherichia coli: the role of recB and recF. Gene. 1991 Jan 2;97(1):131–136. doi: 10.1016/0378-1119(91)90020-c. [DOI] [PubMed] [Google Scholar]
- Paramio J. M., Bauluz C., de Vidania R. Comparative study of the lethal effects of near-UV light (360 nm) and 8-methoxypsoralen plus near-UV on plasmid DNA. Cell Mol Biol. 1991;37(2):125–137. [PubMed] [Google Scholar]
- Pyle L. E., Corcoran L. N., Cocks B. G., Bergemann A. D., Whitley J. C., Finch L. R. Pulsed-field electrophoresis indicates larger-than-expected sizes for mycoplasma genomes. Nucleic Acids Res. 1988 Jul 11;16(13):6015–6025. doi: 10.1093/nar/16.13.6015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roca A. I., Cox M. M. The RecA protein: structure and function. Crit Rev Biochem Mol Biol. 1990;25(6):415–456. doi: 10.3109/10409239009090617. [DOI] [PubMed] [Google Scholar]
- Saxena J. K., Hays J. B., Ackerman E. J. Excision repair of UV-damaged plasmid DNA in Xenopus oocytes is mediated by DNA polymerase alpha (and/or delta). Nucleic Acids Res. 1990 Dec 25;18(24):7425–7432. doi: 10.1093/nar/18.24.7425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seetharam S., Kraemer K. H., Waters H. L., Seidman M. M. Mutational hotspot variability in an ultraviolet-treated shuttle vector plasmid propagated in xeroderma pigmentosum and normal human lymphoblasts and fibroblasts. J Mol Biol. 1990 Apr 5;212(3):433–436. doi: 10.1016/0022-2836(90)90319-H. [DOI] [PubMed] [Google Scholar]
- Sheibani N., Jennerwein M. M., Eastman A. DNA repair in cells sensitive and resistant to cis-diamminedichloroplatinum(II): host cell reactivation of damaged plasmid DNA. Biochemistry. 1989 Apr 4;28(7):3120–3124. doi: 10.1021/bi00433a055. [DOI] [PubMed] [Google Scholar]
- Silberstein Z., Shalit M., Cohen A. Heteroduplex strand-specificity in restriction-stimulated recombination by the RecE pathway of Escherichia coli. Genetics. 1993 Mar;133(3):439–448. doi: 10.1093/genetics/133.3.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smerdon M. J., Bedoyan J., Thoma F. DNA repair in a small yeast plasmid folded into chromatin. Nucleic Acids Res. 1990 Apr 25;18(8):2045–2051. doi: 10.1093/nar/18.8.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. D., Abrahamson R., Minton K. W. Shuttle plasmids constructed by the transformation of an Escherichia coli cloning vector into two Deinococcus radiodurans plasmids. Plasmid. 1989 Sep;22(2):132–142. doi: 10.1016/0147-619x(89)90022-x. [DOI] [PubMed] [Google Scholar]
- Smith M. D., Lennon E., McNeil L. B., Minton K. W. Duplication insertion of drug resistance determinants in the radioresistant bacterium Deinococcus radiodurans. J Bacteriol. 1988 May;170(5):2126–2135. doi: 10.1128/jb.170.5.2126-2135.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. D., Masters C. I., Lennon E., McNeil L. B., Minton K. W. Gene expression in Deinococcus radiodurans. Gene. 1991 Feb 1;98(1):45–52. doi: 10.1016/0378-1119(91)90102-h. [DOI] [PubMed] [Google Scholar]