Abstract
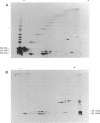
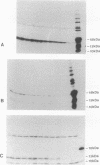
Caedibacter taeniospiralis, an obligate bacterial endosymbiont of Paramecium tetraurelia, confers a killing trait upon its host paramecium. Type 51 R bodies (refractile inclusion bodies) are synthesized by these endosymbionts and are required for expression of the killing trait. The nucleotide sequence of the genetic determinants for type 51 R body synthesis and assembly was determined for C. taeniospiralis 47 and 116. Three independently transcribed genes (rebA, rebB, and rebC) were characterized. To date these are the only genes from C. taeniospiralis to be sequenced and characterized. DNA regulatory regions are recognized by Escherichia coli, and codon usage appears similar to that in E. coli. A fourth open reading frame with appropriate regulatory sequences was found within the reb locus, but no evidence was obtained to suggest that this putative gene is expressed in E. coli. The R body-encoding sequences from both strains are identical. Two-dimensional gel electrophoresis of deletion derivatives shows that two polymerization events are involved in R body assembly. One polymerization event requires only RebB and RebC; the other requires all three proteins. Expression of RebC is necessary for the posttranslational modification of RebA and RebB into species with three and two different molecular weights, respectively. In the presence of RebC, each species of RebB with a different molecular weight has six different isoelectric points.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Supercoiled circular DNA-protein complex in Escherichia coli: purification and induced conversion to an opern circular DNA form. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1159–1166. doi: 10.1073/pnas.62.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilts J. A. Covalently closed, circular DNA in kappa endosymbionts of Paramecium. Genet Res. 1976 Apr;27(2):161–170. doi: 10.1017/s0016672300016360. [DOI] [PubMed] [Google Scholar]
- Hawley D. K., McClure W. R. Compilation and analysis of Escherichia coli promoter DNA sequences. Nucleic Acids Res. 1983 Apr 25;11(8):2237–2255. doi: 10.1093/nar/11.8.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurand A., Rudman B. M., Preer J. R., Jr Prelethal effects of killing action by stock 7 of Paramecium aurelia. J Exp Zool. 1971 Jul;177(3):365–387. doi: 10.1002/jez.1401770311. [DOI] [PubMed] [Google Scholar]
- Kanabrocki J. A., Lalucat J., Cox B. J., Quackenbush R. L. Comparative study of refractile (R) bodies and their genetic determinants: relationship of type 51 R bodies to R bodies produced by Pseudomonas taeniospiralis. J Bacteriol. 1986 Nov;168(2):1019–1022. doi: 10.1128/jb.168.2.1019-1022.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanabrocki J. A., Quackenbush R. L., Pond F. R. Organization and expression of genetic determinants for synthesis and assembly of type 51 R bodies. J Bacteriol. 1986 Oct;168(1):40–48. doi: 10.1128/jb.168.1.40-48.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Marck C. 'DNA Strider': a 'C' program for the fast analysis of DNA and protein sequences on the Apple Macintosh family of computers. Nucleic Acids Res. 1988 Mar 11;16(5):1829–1836. doi: 10.1093/nar/16.5.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Meagher R. B., Tait R. C., Betlach M., Boyer H. W. Protein expression in E. coli minicells by recombinant plasmids. Cell. 1977 Mar;10(3):521–536. doi: 10.1016/0092-8674(77)90039-3. [DOI] [PubMed] [Google Scholar]
- Norrander J., Kempe T., Messing J. Construction of improved M13 vectors using oligodeoxynucleotide-directed mutagenesis. Gene. 1983 Dec;26(1):101–106. doi: 10.1016/0378-1119(83)90040-9. [DOI] [PubMed] [Google Scholar]
- Pond F. R., Gibson I., Lalucat J., Quackenbush R. L. R-body-producing bacteria. Microbiol Rev. 1989 Mar;53(1):25–67. doi: 10.1128/mr.53.1.25-67.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preer J. R., Jr, Preer L. B., Jurand A. Kappa and other endosymbionts in Paramecium aurelia. Bacteriol Rev. 1974 Jun;38(2):113–163. doi: 10.1128/br.38.2.113-163.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preer L. B., Jurand A., Preer J. R., Jr, Rudman B. M. The classes of kappa in Paramecium aurelia. J Cell Sci. 1972 Sep;11(2):581–600. doi: 10.1242/jcs.11.2.581. [DOI] [PubMed] [Google Scholar]
- Quackenbush R. L., Burbach J. A. Cloning and expression of DNA sequences associated with the killer trait of Paramecium tetraurelia stock 47. Proc Natl Acad Sci U S A. 1983 Jan;80(1):250–254. doi: 10.1073/pnas.80.1.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quackenbush R. L., Cox B. J., Kanabrocki J. A. Extrachromosomal elements of extrachromosomal elements of Paramecium and their extrachromosomal elements. Basic Life Sci. 1986;40:265–278. doi: 10.1007/978-1-4684-5251-8_21. [DOI] [PubMed] [Google Scholar]
- Quackenbush R. L., Dilts J. A., Cox B. J. Transposonlike elements in Caedibacter taeniospiralis. J Bacteriol. 1986 Apr;166(1):349–352. doi: 10.1128/jb.166.1.349-352.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quackenbush R. L. Plasmids from bacterial endosymbionts of hump-killer paramecia. Plasmid. 1983 May;9(3):298–306. doi: 10.1016/0147-619x(83)90007-0. [DOI] [PubMed] [Google Scholar]
- Roozen K. J., Fenwick R. G., Jr, Curtiss R., 3rd Synthesis of ribonucleic acid and protein in plasmid-containing minicells of Escherichia coli K-12. J Bacteriol. 1971 Jul;107(1):21–33. doi: 10.1128/jb.107.1.21-33.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R. The use of thin acrylamide gels for DNA sequencing. FEBS Lett. 1978 Mar 1;87(1):107–110. doi: 10.1016/0014-5793(78)80145-8. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp P. M., Li W. H. Codon usage in regulatory genes in Escherichia coli does not reflect selection for 'rare' codons. Nucleic Acids Res. 1986 Oct 10;14(19):7737–7749. doi: 10.1093/nar/14.19.7737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stormo G. D., Schneider T. D., Gold L. M. Characterization of translational initiation sites in E. coli. Nucleic Acids Res. 1982 May 11;10(9):2971–2996. doi: 10.1093/nar/10.9.2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]