Abstract
We recently reported that a germ-line insertion in the rat homologue of the human tuberous sclerosis gene (TSC2) gives rise to dominantly inherited cancer in the Eker rat model. In this study, we constructed transgenic Eker rats with introduction of a wild-type Tsc2 gene to ascertain whether suppression of the Eker phenotype is possible. Rescue from embryonic lethality of mutant homozygotes (Eker/Eker) and suppression of N-ethyl-N-nitrosourea-induced renal carcinogenesis in heterozygotes (Eker/+) were both observed, defining the germ-line Tsc2 mutation in the Eker rat as embryonal lethal and tumor predisposing mutation. To the best of our knowledge, this is the first report of rescue from a naturally occurring dominantly inherited cancer. This transgenic rescue system will be useful to analyze Tsc2 gene function, its relation to tumorigenesis in vivo, and genetic–environmental interactions in carcinogenesis.
Keywords: hereditary cancer, two-hit model, tumor suppressor gene, animal model, gene therapy
The hereditary renal carcinoma (RC) in the rat, originally reported by Eker (1) in 1954, is an excellent example of a Mendelian dominantly inherited predisposition for development of a specific cancer in an experimental animal. Recently, we and others have identified a germ-line mutation of the tuberous sclerosis (Tsc2) gene in the Eker rat (2–5), suggesting it to be a novel tumor suppressor gene fitting Knudson’s two-hit hypothesis (6, 7). At the histological level, RCs develop through multiple stages from early preneoplastic lesions (e.g., phenotypically altered tubules) to adenomas in virtually all heterozygotes by the age of 1 year (6). The homozygous mutant condition is lethal at around 13 days of fetal life (6). The Eker rat is in addition highly susceptible to induction of RCs (but not Wilms’ tumors) by transplacental administration of N-ethyl-N-nitrosourea (ENU) (8).
The detection of loss of the wild-type allele, even in the earliest preneoplastic lesions, supports the hypothesis that a second somatic mutation is the rate-limiting step for renal carcinogenesis in the Eker rat model (9, 10). Loss of heterozygosity of the TSC2 locus is also observed in hamartomas from tuberous sclerosis patients (11–13). Recent demonstrations of growth suppression of Eker rat RC cells by wild-type Tsc2 gene transduction provided further evidence of the tumor suppressor nature of the Tsc2 gene (14, 15)
The Tsc2 product (tuberin) contains a short amino acid sequence homology to Rap1 GTPase-activating protein conserved in human and rat (5, 16). Wienecke et al. (17, 18) reported that tuberin has weak GTPase-activating protein activity for Rap1a and localizes to Golgi apparatus. Other potential functional domains include two transcriptional activation domains (AD1 and AD2) in the carboxyl terminus of the Tsc2 gene product (19), a zinc-finger-like region (our unpublished observation), and a potential src-homology 3 region (SH3) binding domain (20). However, the function of tuberin is not yet fully understood.
Human tuberous sclerosis is an autosomal dominant genetic disease characterized by phacomatosis with manifestations that include mental retardation, seizures, and angiofibroma, although abortive types are more frequent. The phenotype in humans differs from that in the Eker rat, except for the occurrence of RCs (in humans, angiomyolipomas are more common) (3). Given the lack of knowledge regarding the molecular mechanism underlying human tuberous sclerosis, the potential of the Eker rat for elucidating the TSC2/Tsc2 gene role in renal carcinogenesis, as well as for studying species-specific differences in tumorigenesis and/or cell-type-specific carcinogenesis deserved stress.
In this study, to confirm that a tumor predisposition in the Eker rat is caused by the Tsc2 germ-line mutation and to establish in vivo system for analysis Tsc2 gene role, we constructed transgenic Eker rats with introduction of a chimeric minigene consisting of a Tsc2 cDNA and its 5′-upstream promoter region.
MATERIALS AND METHODS
Construction of a Wild-Type Tsc2 Transgene (Tg).
A ≈3-kb SpeI–BglII fragment covering the 710 nt of 5′-upstream region (5′UR) to intron 1 and a ≈0.1-kb BglII fragment covering the 3′ part of intron 1 to the 5′ part of exon 2 of the rat Tsc2 gene were prepared from a cosmid clone, cosTsc2-1 (16). To obtain the Tsc2 cDNA fragment containing the Brown Norway strain type polymorphic sequence in exon 30 (21), reverse transcription–PCR (RT-PCR) was performed using kidney total RNA from a Brown Norway strain animal (Charles River Breeding Laboratory) using the primer set, RTSC8 (5′-GCTCAGCATCAAGCTCTGAT-3′, forward) and RTSC21 (5′-AGGAGATGGCCCGCTCAAT-3′, reverse). A 5.2-kb BglII–XhoI Tsc2 cDNA covering the 3′ part of exon 2 to the poly(A) tail, lacking exons 25 and 31 but including the Brown Norway type polymorphic sequence, was constructed using the cDNA fragment obtained by RT-PCR described above and several cDNA clones from the Long Evans strain (Kiwa Breeding Laboratory, Japan) (16). The wild-type Tsc2 Tg was constructed in the SpeI–XhoI sites of pBluescript SK(−) using these genomic and cDNA fragments and a ≈150 bp fragment containing the simian virus 40 (SV40) poly(A) addition signal derived from pSG5 (22) after sequential subcloning. The resulting plasmid was named pMGTsc2. All subclonings were confirmed by sequence analysis. For injection, pMGTsc2 DNA was linearized by complete digestion of NotI. Then it was partially digested with KpnI and the fragment (≈8.5 kb) containing the full-length Tg without the plasmid sequence was prepared with a GENECLEAN II Kit (Bio 101) after separation by SeaKem agarose (FMC BioProducts) gel electrophoresis.
Generation of Transgenic Founders.
Female 8- to 13-week-old Wistar rats (Charles River Breeding Laboratory) at metestrus were stimulated to superovulate by i.p. injection of 150 units/kg pregnant mare’s serum gonadotropin (PMS-ZENYAKU; Nippon Zenyaku, Japan) followed by 75 units/kg human chorionic gonadotropin (PUBEROGEN; Sankyo) 48–50 hr later, and were mated with male Eker carriers. At 32 hr after the human chorionic gonadotropin injection, the oviducts were removed surgically and prenuclear stage eggs were collected by flushing with TC medium (23) containing 0.1% hyaruronidase (Sigma type I-S) for 5 min to remove cumulus cells. The eggs were washed three times with TC medium and then stored at 37°C under 5% CO2/95% air at saturation humidity until use. Tsc2 Tg DNA [5 μg/ml in 10 mM Tris·HCl (pH 7.6) containing 0.1 mM EDTA] was microinjected into single male pronuclei according to the method of Hochi et al. (24). The eggs were subsequently cultured overnight and transferred to oviducts of recipient female Wistar rats on day 1 of pseudopregnancy after mating with vasectomized male rats.
Transplacental ENU Treatment and Histological Analysis.
Normal female Wistar rats were mated with male Eker rats carrying the Tg and given a single i.p. carcinogenic dose (80 mg/kg of body weight) of ENU (Nacalai Tesque, Kyoto) on the 15th day of gestation (8). At 8 weeks after birth, litters from these females were sacrificed and their kidneys were analyzed. For histological analysis, kidney tissues were fixed in 10% formalin, and a routine histological examination was carried out on hematoxylin/eosin-stained paraffin sections.
Southern Blot Analysis.
DNAs were isolated from rat tails by proteinase K digestion followed by phenol/chloroform extraction (6). Ten micrograms of each DNA sample was digested with BamHI and separated by 1% agarose gel electrophoresis. After transfer to nylon membranes (Biodyne B; Pall Biosupport) under alkaline conditions (0.4 M NaOH), prehybridization, hybridization, and washing were performed as described (3). The probe used was a 0.7-kb EcoRI subfragment of a rat Tsc2 cDNA (7a1) covering exons 21–27, except for exon 25 (16). Estimation of Tg copy numbers were performed by Southern blot analysis using Tg plasmid as amount markers.
RNA Analysis.
Total RNAs were isolated by the guanidium-isothiocyanate/phenol extraction method and then poly(A)+ RNAs were selected using oligo(dT) latex beads (Nippon Roche, Tokyo). Northern blot analysis was performed as discribed (3). An ≈150-bp fragment containing a SV40 poly(A)+ addition signal (22) was used as a probe to detect the Tg-specific transcripts. For RT-PCR analysis, 5 μg aliquots of poly(A)+ RNAs was used as templates for the reverse transcription with random primers in 20 μl reaction mixtures. Then, 1 μl samples were subjected to PCR in 25 μl reaction mixtures containing 10 mM Tris·HCl (pH 8.8), 50 mM KCl, 200 μM of each dNTP, 1 mM MgCl2, 0.1% Triton X-100, 2 units of Taq polymerase (Toyobo, Osaka), and 25 pmol of each primer [RTSC16, 5′-AGCCTATGTGCCTTTGCTGA-3′ (forward), and RTSC19, 5′-CGAGTCTGTGTTAGAACGTG-3′ (reverse)]. Amplification was achieved with 1 cycle for 3 min at 94°C, 35 cycles for 1 min at 94°C, 1 min at 55°C, 1.5 min at 72°C, and 1 cycle for 3 min at 72°C. After amplification, 1 μl aliquots were digested with RsaI and separated by 3% Nusieve-GTG gel (FMC BioProducts) electrophoresis.
RESULTS AND DISCUSSION
Establishment of Wild-Type Tsc2 Transgenic Founder Rats.
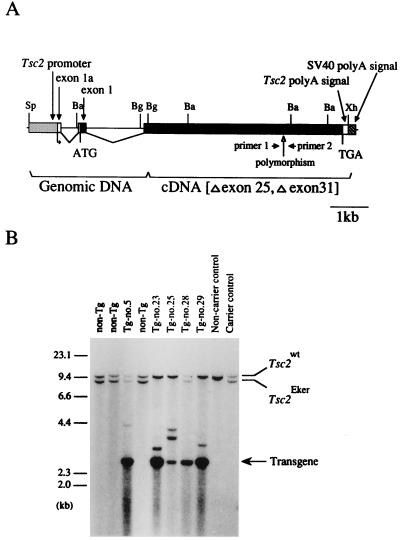
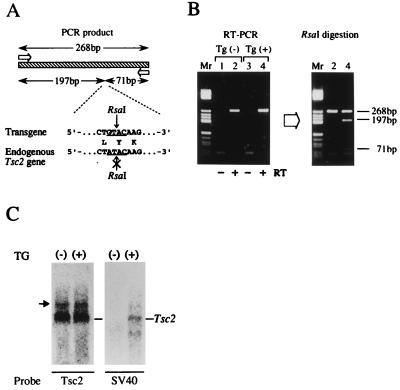
We constructed a wild-type rat Tsc2 Tg consisting of a genomic DNA fragment covering 710-bp 5′UR to exon 2, a Tsc2 cDNA that lacks alternatively spliced exons, exons 25 and 31 (16), and the SV40 poly(A) addition signal sequence (Fig. 1A). The 710-bp 5′UR used here shows promoter activity in vitro in various cells (T.K. and O.H., unpublished data). To detect mRNA from this Tg, a Brown Norway strain-type polymorphic sequence in exon 30, which can be distinguished by RsaI digestion, was introduced (21). By injection of this Tg into fertilized eggs from normal female Wistar rats mated with male Eker carriers [harboring the mutant-type Tsc2 allele (Tsc2Eker)], we obtained five transgenic founders (Tg-no.5, no.23, no.25, no.28, and no.29; Fig. 1B), two being Eker carriers (Tg-no.5, male carrying eight copies of Tg and Tg-no.28, female carrying three copies of Tg) which could be extensively characterized. Expression of Tg was analyzed by RT-PCR and Northern blot analysis (Fig. 2). By RsaI digestion after RT-PCR amplification, Tg-specific cDNA fragments were detected in the Tg-carrying offspring kidneys (Fig. 2 A and B). Tg-specific, ≈5.5-kb transcripts, the same size as the internal Tsc2 mRNA, were also detected in Tg-carrying rats by Northern blot analysis (Fig. 2C). Thus, the wild-type Tsc2 Tg used here was actually expressed in vivo.
Figure 1.
Generation of the transgenic founders. (A) Structure of the wild-type Tsc2 gene used in this study. Closed and opened boxes indicate coding and noncoding regions of the Tsc2 gene lacking exons 25 and 31 due to alternative splicing, respectively. Hatched and striped boxes indicate the Tsc2 promoter region and the SV40 DNA fragment containing the poly(A) addition signal, respectively. Positions of the translational initiation (ATG) and termination (TGA) codons, polymorphic sequence, and primers for RT-PCR analysis are noted below. Sp, SpeI; Ba, BamHI; Bg, BglII; Xh, XhoI. (B) Southern blot analysis of the transgenic founders. BamHI-digested DNA samples from five transgenic founders (Tg-no.5, no.23, no.25, no.28, and no.29), three nontransgenic littermates (non-Tg), an Eker carrier, and noncarrier controls were probed with a 32P-labeled rat Tsc2 cDNA fragment covering exons 21–27. Band positions of the endogenous wild-type Tsc2 allele (Tsc2wt), the endogenous germ-line mutant Tsc2 allele (Tsc2Eker), and the Tg are indicated on the right. Positions of size markers (λ/HindIII fragments) are shown on the left.
Figure 2.
Expression of Tg. (A) Schematic representation of the RT-PCR analysis of Tg expression. The open arrows and striped box indicate primers and the amplified product (268 bp), respectively. Nucleotide and amino acid sequences of the polymorphic site are shown below and the RsaI recognition sequence in the Tg and the corresponding region of the endogenous gene are underlined. RsaI digestion divides the Tg mRNA, but not the endogenous gene, into two fragments (197 bp and 71 bp). (B) Results of RT-PCR analysis. (Left) Results of PCR amplification for kidney RNAs from two F1 offspring of Tg-no.28. Lanes 1 and 2, and 3 and 4 are offspring without the Tg [Tg(−)] and with the Tg [Tg(+)], respectively. Lanes 1 and 3 are negative controls without RT. (Right) Results of RsaI digestion of amplified products. The sizes of the bands are shown on the right. Lane Mr, size marker (pBR322/HaeIII fragments). (C) Northern blot analysis. Equal amount (10 μg) of total kidney RNAs from offsprings of Tg-no.28 with (+) or without (−) Tg are probed with Tsc2 cDNA (Tsc2) or a fragment containing SV40 poly(A) addition signal. The arrow indicates aberrant Tsc2 transcript derived from Eker mutant allele.
Rescue from Embryonic Lethality by Wild-Type Tsc2 Tg.
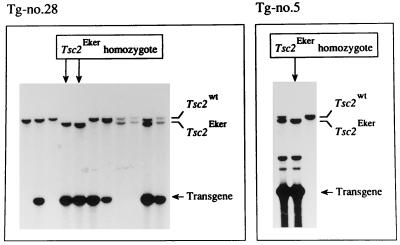
To examine the effects of extra-copies of the wild-type Tsc2 gene on embryonic lethality in Tsc2Eker homozygotes, Tg-no.5 and Tg-no.28 were mated with two female and one male Eker carriers, respectively, and the Tsc2 genotypes of their offspring were determined. If Tg can complement the defects caused by lack of Tsc2 function, Tsc2Eker homozygotes would be expected to develop normally. Indeed, we obtained offspring showing the Tsc2Eker homozygosity with transmission of Tg in all three cross matings (Fig. 3). The incidences of Tsc2Eker homozygotes were 1/13 (7.6%), 1/14 (7.1%), and 2/11 (18.2%) with the two Tg-no.5 crosses and the one Tg-no.28 cross, respectively (total, 4/38 = 10.5%). These results were not significantly different from expected (1/7, 14.3%), assuming that Tg in a single genomic site (other than rat chromosome 10, which bears the Tsc2 gene) segregates randomly according to Mendelian rules. We also obtained Tsc2Eker homozygous offspring by intercrossing Tg-carrying littermates derived from Tg-no.29 (data not shown). The findings thus indicate that extra copies of the wild-type Tsc2 gene effect rescue from the embryonic lethality caused by Tsc2Eker homozygosity.
Figure 3.
Rescue from Tsc2Eker homozygous lethality by introduction of the wild-type Tsc2 Tg. Southern blot analysis was performed after BamHI digestion as for Fig. 1B. The results with all littermates from the Tg-no.28 × male Eker carrier cross (Left) and three representative littermates from a Tg-no.5 × female Eker carrier cross (Right) are shown. In each panel, band positions of the endogenous wild-type Tsc2 allele (Tsc2wt), the endogenous germ-line mutant Tsc2 allele (Tsc2Eker), and the Tg are indicated on the right. Lanes of rescued Tsc2Eker homozygotes are indicated with arrows above.
Suppression of Chemically Induced Renal Carcinogenesis by Wild-Type Tsc2 Tg.
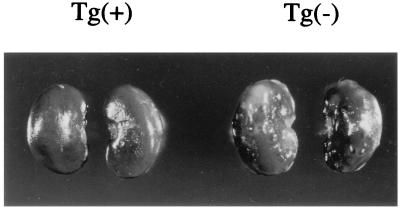
We also examined the influence of extra copies of the wild-type Tsc2 gene on renal carcinogenesis in the Eker rat. As the spontaneous formation of gross renal tumors takes over 1 year, we used ENU for lesion induction (8). After transplacental administration of ENU on the 15th day of gestation, Eker carrier offspring develop multiple, bilateral renal tumors by 8 weeks after birth whereas non-Eker carriers do not. We mated normal female Wistar rats with Tg-no.5 founder or two Tg-carrying male Eker carriers derived from Tg-no.28, and treated them with ENU. At 8 weeks after birth, we macroscopically observed neoplastic lesions in the kidneys of all Eker carrier offspring without Tg (8 cases), confirming the effectiveness of the ENU treatment (Fig. 4). Histological examination revealed multiple renal tumors associated with cystic lesions (ref. 8 and data not shown). In contrast, kidneys from Tg-carrying Eker littermates (28 cases) did not show any neoplastic lesions, even on histological examination (Fig. 4 and data not shown). The results indicate that extra copies of the wild-type Tsc2 gene suppress ENU induction of renal tumors in the Eker rat carriers. Complete loss of tumor suppressor function of the Tsc2 gene could be prevented by Tg even if a second hit on the endogenous wild-type Tsc2 allele occurred. As in the case of pituitary tumor development in the retinoblastoma gene knockout (Rb−/Rb−) mice carrying wild-type human RB Tg (25), it remains to be seen if rescued Tsc2Eker/Tsc2Eker rats may develop renal tumors by loss of Tg in later life.
Figure 4.
Suppression of ENU-induced renal carcinogenesis by introduction of the wild-type Tsc2 Tg. Kidneys from two female F2 Eker carriers from Tg-no.28 with [Left, Tg(+)] or without [Right, Tg(−)] the Tg were examined at 8 weeks after birth. Note that the Tg(−) kidneys exhibit multiple, bilateral lesions whereas Tg(+) kidneys do not.
The data presented in this study clearly confirm the tumor suppressor nature of the Tsc2 gene and define the germ-line Tsc2 mutation that is present in the Eker rat as a tumor predisposing and embryonic lethal mutation. For generation of Tg, we used a cDNA lacking exons 25 and 31. Alternative splicing events involving these two exons are evolutionally conserved in humans, the mouse, and pufferfish, suggesting that they might be important for the Tsc2 gene function (26–28). However, their lack did not prevent the complementation activity of Tg in the present studies. Thus, exons 25 and 31 are not necessary, at least for tumor suppressor function and rescue from the embryonic lethality in Tsc2Eker homozygotes. However, the Eker rat develops extra-renal tumors, albeit with a less complete penetrance as in the RC case (29), and exons 25 and 31 may have some relevance here. Thus, it is clearly of interest whether these tumors are also suppressed in Tg-carrying Eker carriers. As our Tg function in vivo, its expression driven by 710-bp 5′UR may be almost identical to that of the endogenous Tsc2 gene. However, subtle differences may exist caused by the lack of transcriptional regulatory elements in Tg. In rescued Tsc2Eker homozygotes, abnormalities might develop in later life (25). Longer term phenotypic analysis of rescued Tsc2Eker homozygotes and examination of the expression profile of Tg at histological level therefore holds promise for clarification of various aspects of Tsc2 function.
Acknowledgments
We gratefully acknowledge Ms. E. Kobayashi for her help. We would also like to thank Drs. Haruo Sugano, Tomoyuki Kitagawa, and Alfred G. Knudson for their encouragement throughout this work. The research was supported in part by Grants-in-Aid for Cancer Research from the Ministry of Education, Science and Culture of Japan and the Council for Tobacco Research of the United States.
ABBREVIATIONS
- ENU
N-ethyl-N-nitrosourea
- RC
renal carcinoma
- Tg
transgene
- 5′UR
5′-upstream region
- SV40
simian virus 40
- RT-PCR
reverse transcription–PCR
References
- 1.Eker R, Mossige J A. Nature (London) 1961;189:858–859. [Google Scholar]
- 2.Hino O, Kobayashi T, Tsuchiya H, Kikuchi Y, Kobayashi E, Mitani H, Hirayama Y. Biochem Biophys Res Commun. 1994;203:1302–1308. doi: 10.1006/bbrc.1994.2324. [DOI] [PubMed] [Google Scholar]
- 3.Kobayashi T, Hirayama Y, Kobayashi E, Kubo Y, Hino O. Nat Genet. 1995;9:70–74. doi: 10.1038/ng0195-70. [DOI] [PubMed] [Google Scholar]
- 4.Yeung R S, Xiao G-H, Jin F, Lee W-C, Testa J R, Knudson A G. Proc Natl Acad Sci USA. 1994;91:11413–11416. doi: 10.1073/pnas.91.24.11413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.The European Chromosome 16 Tuberous Sclerosis Consortium. Cell. 1993;75:1305–1315. doi: 10.1016/0092-8674(93)90618-z. [DOI] [PubMed] [Google Scholar]
- 6.Hino O, Klein-Szanto A J P, Freed J J, Testa J R, Brown D Q, Vilensky M, Yeung R S, Tartof K D, Knudson A G. Proc Natl Acad Sci USA. 1993;90:327–331. doi: 10.1073/pnas.90.1.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Knudson A G. Proc Natl Acad Sci USA. 1971;68:820–823. doi: 10.1073/pnas.68.4.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hino O, Mitani H, Knudson A G. Cancer Res. 1993;53:5856–5858. [PubMed] [Google Scholar]
- 9.Kubo Y, Mitani H, Hino O. Cancer Res. 1994;54:2633–2635. [PubMed] [Google Scholar]
- 10.Kubo Y, Klimek F, Kikuchi Y, Bannasch P, Hino O. Cancer Res. 1995;55:989–990. [PubMed] [Google Scholar]
- 11.Green A J, Smith M, Yates J R W. Nat Genet. 1994;6:193–196. doi: 10.1038/ng0294-193. [DOI] [PubMed] [Google Scholar]
- 12.Henske E P, Neumann H P H, Scheithauser B W, Herbst E W, Short M P, Kwiatkowski D J. Genes Chromosome Cancer. 1995;13:295–298. doi: 10.1002/gcc.2870130411. [DOI] [PubMed] [Google Scholar]
- 13.Carbonara C, Longa L, Grosso E, Mazzucco G, Borrone C, Garre M L, Brisgotti M, Filippi G, Scabar A, Giannotti A, Falzoni P, Monga G, Garini G, Gabrielli M, Riegler P, Danesino C, Ruggieri M, Margo G, Migone N. Genes Chromosome Cancer. 1996;15:18–25. doi: 10.1002/(SICI)1098-2264(199601)15:1<18::AID-GCC3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 14.Orimoto K, Tsuchiya H, Kobayashi T, Matsuda T, Hino O. Biochem Biophys Res Commun. 1996;219:70–75. doi: 10.1006/bbrc.1996.0183. [DOI] [PubMed] [Google Scholar]
- 15.Jin F, Wienecke R, Xiao G-H, Maize J C, DeClue J E, Yeung R S. Proc Natl Acad Sci USA. 1996;93:9145–9159. doi: 10.1073/pnas.93.17.9154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kobayashi T, Nishizawa M, Hirayama Y, Kobayashi E, Hino O. Nucleic Acids Res. 1995;23:2608–2613. doi: 10.1093/nar/23.14.2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wienecke R, Konig A, DeClue J E. J Biol Chem. 1995;270:16409–16414. doi: 10.1074/jbc.270.27.16409. [DOI] [PubMed] [Google Scholar]
- 18.Wienecke R, Maize J C, Shoarinejad F, Vass W C, Reed J, Bonifacino J S, Resau J H, de Gunzburg J, Yeung R S, DeClue J E. Oncogene. 1996;13:915–923. [PubMed] [Google Scholar]
- 19.Tsuchiya H, Orimoto K, Kobayashi T, Hino O. Cancer Res. 1996;56:429–433. [PubMed] [Google Scholar]
- 20.Olsson P G, Schofield J N, Edwards Y H, Frischauf A M. Mamm Genome. 1996;7:212–215. doi: 10.1007/s003359900057. [DOI] [PubMed] [Google Scholar]
- 21.Kubo Y, Kikuchi Y, Mitani H, Kobayashi E, Kobayashi T, Hino O. Jpn J Cancer Res. 1995;86:828–832. doi: 10.1111/j.1349-7006.1995.tb03092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Green S, Issemann I, Sheer E. Nucleic Acids Res. 1988;16:369. doi: 10.1093/nar/16.1.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Toyoda Y, Chang M C. J Reprod Fertil. 1974;36:9–22. doi: 10.1530/jrf.0.0360009. [DOI] [PubMed] [Google Scholar]
- 24.Hochi S, Ninomiya T, Honma M, Yuki A. Anim Biotechnol. 1990;1:175–184. [Google Scholar]
- 25.Chang C-Y, Riley D J, Lee E Y-H P, Lee W-H. Cell Growth Differ. 1993;4:1057–1064. [PubMed] [Google Scholar]
- 26.Xiao G-H, Jin F, Yeung R S. Cell Growth Differ. 1995;6:1185–1191. [PubMed] [Google Scholar]
- 27.Xu L, Sterner C, Maheshwar M M, Wilson P J, Nellist M, Short P M, Haines J L, Sampson J R, Ramesh V. Genomics. 1995;27:475–480. doi: 10.1006/geno.1995.1079. [DOI] [PubMed] [Google Scholar]
- 28.Maheshwar M M, Sandford R, Nellist M, Cheadle J P, Sgotto B, Vaudin M, Sampson J R. Hum Mol Genet. 1996;5:131–137. doi: 10.1093/hmg/5.1.131. [DOI] [PubMed] [Google Scholar]
- 29.Hino O, Mitani H, Katsuyama H, Kubo Y. Cancer Lett. 1994;83:117–121. doi: 10.1016/0304-3835(94)90307-7. [DOI] [PubMed] [Google Scholar]