Abstract
We determined the cytotoxicity of AG490 as a single agent and in combination with 7-OH-hydroxystaurosporine (UCN-01) in a panel of malignant human glioma cell lines. Because p53 has important roles in cell cycle checkpoints, it has been anticipated that modulation of checkpoint pathways should sensitize p53-defective cells while sparing the normal cells. Cell proliferation was determined from dose-response curves. AG490 was effective as a cytotoxic agent alone regardless of p53 status. Combining the Chk1 inhibitor UCN-01 dramatically enhanced the response to AG490 in p53 mutated or deleted glioma cells. An opposite effect was noted in p53 wild-type cells, in which UCN-01 and AG490 had antagonist effects on cell proliferation and viability. We found that AG490 enhanced BAD phosphorylation in p53 wild type glioma cells, which appeared to protect against UCN-01 induced cytotoxicity, whereas AG490 enhances UCN-01-induced cytotoxicity in p53-defective cell lines by suppression of BAD phosphorylation, and induction of BAX and PARP cleavage. These observations highlight the potential for genotype-dependent factors to strongly influence response to signaling-targeted therapies in malignant gliomas and the importance of considering such factors in correlative response analyses for these agents.
Keywords: Synergy, AG490, UCN01, glioma, p53, BAD, BAX, apoptosis
1. Introduction
Although malignant gliomas are commonly treated with a combination of radiation and chemotherapy, the majority of tumors recur rapidly after conventional therapy [1, 2]. However, response to treatment varies widely, reflecting differences between tumors in their vulnerability to undergoing apoptosis in response to cytotoxic treatment modalities. Proliferating cells, including tumor cells, respond to genotoxic stress by triggering a series of signaling events known as cell cycle checkpoints, which function to delay cell cycle progression, thereby facilitating repair of genetic insults [3]. Tumor cells commonly exhibit mutations in genes that can either counteract or enhance checkpoint function [4]. Cells that progress through the cell cycle despite having undergone DNA damage can either undergo apoptosis or survive with increasingly aberrant genetic features. Thus, abrogation of checkpoint function can have unpredictable consequences, potentially increasing the lethality of some insults and favoring increased anaplasia in response to others. The tumor suppressor gene p53 has been observed to play an important role in G1 cell cycle arrest and apoptosis [5]. Some studies have demonstrated that cells lacking p53 or having a mutated p53 are more resistant to cytotoxic therapies [6, 7]. The concept of enhancing the cytotoxicity of DNA-damaging agents by checkpoint inhibition was first exemplified by caffeine [8], an inhibitor of both ataxia-telangectasia (ATM) and ataxia-telangectasia-related (ATR) [9], and more recently shown with 7-hydroxystaurosporine (UCN-01), an anticancer agent in Phase II clinical trials.
UCN-01 enhances the sensitivity of cancer cells to radiation and chemotherapeutic agents by abrogating DNA damage induced checkpoints [10, 11] by targeting the Cdc25C-Cdc2 regulatory pathway [12]. UCN-01, at nontoxic concentrations, abrogates both the S and G2/M checkpoints and potentiates the cytotoxic effects of a wide spectrum of DNA-damaging agents, including ionizing radiation [13], cisplatin [14], temozolomide [15], and camptothecin [11]. There has been conflicting data as to whether UCN-01 selectively enhances the cytotoxicity of DNA-damaging agents in cells with nonfunctional p53 [11, 16].
To identify whether UCN-01 could potentiate antiproliferative or cytotoxic activity of various conventional chemotherapeutic agents or other signaling inhibitors in glioma cells, we performed a series of combinatorial assays in the T98G and U87 glioma cell lines. These studies showed a striking degree of synergism between UCN-01 and the janus kinase/signal transducers and activators of transcription (JAK/STAT) inhibitor, AG490, in p53 defective, but not in wild type cell lines. The JAK/STAT pathway was originally discovered as an effector of normal IFN signaling. However, several recent studies (reviewed in ref [17]) have demonstrated that STAT proteins are involved in signaling by many growth factor receptors known to be dysregulated in gliomas [18], and that constitutively activated STAT signaling contributes to cell proliferation and resistance to apoptosis in a variety of tumor types [19, 20].
Because UCN-01 and JAK/STAT pathway inhibitors interfere with survival signaling by distinct mechanisms, we reasoned that the combination of these agents might cooperate to block tumor cell proliferation and induce apoptosis. In the current study, we examined the effect of UCN-01 and AG490 for inhibiting glioma cell proliferation in vitro using a genetically diverse panel of malignant glioma cell lines. Interestingly, we observed that AG490 induced BAD phosphorylation and suppression of UCN-01-induced apoptosis in p53 wild type cells whereas growth inhibition and apoptosis was potentiated in p53 defective cells. These observations call attention to the importance of understanding the profile of genotypic alterations in individual tumors, which can strongly influence response to signaling-targeted therapies.
2. Materials and Methods
2.1. Cell Culture
The established malignant glioma cell lines U87, T98G, A172, human pulmonary fibroblasts, and human umbilical vein endothelial cells (HUVEC) were obtained from the American Type Culture Collection. Human astrocytes and human cerebellar astrocytes were obtained from ScienCell Research Laboratories, San Diego, CA. LN18, LNZ308, and LNZ428 were generously provided by Dr. Nicolas de Tribolet. U87, T98G and human pulmonary fibroblasts were cultured in growth medium composed of minimum essential medium supplemented with sodium pyruvate and non-essential amino acids; A172, LN18, LNZ308, and LNZ428 in α-minimal essential medium supplemented with L-glutamine; human astrocytes in Astrocyte Growth Medium; and HUVEC in Endothelial Cell Medium (ScienCell Research Laboratories). All growth media contained 10% fetal calf serum, L-glutamine, 100 IU/ml penicillin, 100 mg/ml streptomycin and 0.25 mg/ml amphotericin (Life Technologies, Inc., Bethesda, MD). Cells were grown in 75-cm2 flasks at 37°C in a humidified atmosphere with 5% carbon dioxide and were subcultured every 3 days by treatment with 0.25% trypsin in Hanks' balanced salt solution (Life Technologies, Inc.).
The p53 status of each glioma cell line has been established previously as follows: p53 wild type, U87, and A172; p53 deleted, LNZ308; and p53 defective, T98G, LN18, and LNZ428 [21].
2.2. Inhibitors and Reagents
AG490, U0126 and LY294002 were purchased from Calbiochem. UCN-01 was provided by Dr. Edward Sausville (Development Therapeutics Program, National Cancer Institute). Materials were dissolved in sterile DMSO and stored frozen under light-protected condition at −20°C.
2.3. Cell Proliferation Assay
Cells (5 X 103/well) were plated in 96-well microtiter plates in 100μl of growth medium, and after overnight attachment, were exposed for 3 days to different concentrations of AG490 and UCN-01, alone and in combination. Control cells received vehicle alone. After the treatment interval, cells were washed in inhibitor-free medium and the number of viable cells was determined using a colorimetric cell proliferation assay (CellTiter96 Aqueous Non-Radioactive Cell Proliferation Assay; Promega, Madison, WI), which measures the bioreduction of MTS (3-[4,5-dimethylthiazol-2yl]-5-[3-carboxymethoxyphenyl]-2-[4-sulfophenyl]-2H tetrazolium) by dehydrogenase enzymes of metabolically active cells into a soluble formazan product, in the presence of the electron coupling reagent phenazine methosulfate (PMS) [22]. To perform the assay, 20 μl of MTS/PMS solution was added to each well; after 1 h of incubation at 37°C, absorbance was measured at 490 nM in a microplate reader. Triplicate wells with predetermined cell numbers were subjected to the above assay in parallel with the test samples to normalize the absorbance readings. All studies were conducted in triplicate and repeated at least three times independently.
2.4. Annexin V Apoptosis Assay
Apoptosis induction in control (DMSO-treated) or inhibitor-treated cells was assayed by the detection of membrane externalization of phosphatidylserine with Annexin V-FITC conjugate using an Annexin V assay kit according to manufacturer's protocol (Molecular Probes). Briefly, 2 X 105 cells were harvested at various intervals after treatment and washed twice with ice-cold phosphate-buffered saline (PBS) and resuspended in 200 μl of binding buffer. Both adherent and floating cells were harvested for the apoptosis assay. Annexin V-FITC and propidium iodide (1 μg/ml) were added to individual samples and incubated for 15 min in a dark environment. The reaction was stopped by adding 300 μl of 1 X binding buffer. Then the cells were analyzed by flow cytometry with FACSCalibur Flow Cytometer (Beckton Dickinson).
2.5. Cell Cycle Analysis
The effect of varying concentrations of inhibitors on cell cycle distribution was determined by flow cytometric analysis of the DNA content of cell nuclei following staining with propidium iodide. Briefly, cells grown exponentially to 40−50% confluency were exposed to the inhibitors or DMSO for a range of intervals, harvested, washed briefly in ice-cold PBS, and fixed in 70% ethanol. DNA was stained by incubating the cells in PBS containing propidium iodide (50 μg/ml) and RNase A (1 mg/ml) for 60 min at room temperature, and fluorescence was measured and analyzed using a Becton Dickinson FACScan and the Cell Quest software (Becton Dickinson Immunocytometry Systems, San Jose, CA).
2.6. Western blotting Analysis
Treated and untreated cells were washed in cold PBS and lysed in buffer containing 30 mM Hepes, 10% glycerol, 1% Triton X-100, 100 mM NaCl, 10 mM MgCl2, 5 mM EDTA, 2 mM Na3VO4, 2 mM ß-glycerophosphate, 1 mM PMSF, 1 mM AEBSF, 0.8 μM Aprotinin, 50 μM Bestatin, 15 μM E-64, 20 μM Leupeptin, and 10 μM Pepstatin A, for 15 minutes on ice. Samples were centrifuged at 12,000 X g for 15 min, supernatants were isolated, and protein was quantified using Protein Assay Reagent (Pierce, Rockford, IL). Equal amounts of protein were separated by sodium dodecyl sulfatepolyacrylamide gel electrophoresis (SDS-PAGE), and electrotransferred onto a nylon membrane (Invitrogen, Carlsbad, CA). Non-specific antibody binding was blocked by incubation of the blots with 2% bovine serum albumin (BSA) in Tris-buffered saline (TBS)-Tween 20 (0.1%) for 1 h at room temperature. The blots were then probed with appropriate dilutions of primary antibody overnight at 4 °C. The antibody-labeled blots were washed three times in TBS-Tween 20 for 15 min and then incubated with a 1:1500 dilution of horseradish peroxidase-conjugated secondary antibody (Santa Cruz Biotechnology, Santa Cruz, CA) in TBS-Tween 20 at room temperature for 1 h. After additional washing in TBS-Tween 20, the proteins were visualized by Western Blot Chemiluminescence Reagent (Cell Signaling Technology). The primary antibodies were obtained from Cell Signaling Technologies.
2.7. Analysis of Combinatorial Effects
The significance of differences between experimental conditions was determined using the 2-tailed Student t test. To characterize IC50 concentrations and synergistic effects between the agents, a commercially available software program was used [23] (Calcusyn; Biosoft, Ferguson, MO)
3. Results
3.1. Growth Inhibition of AG490
We first examined the effect of AG490 as a single agent on the cellular proliferation of a panel of glioma cell lines. Cells were cultured with increasing concentrations of AG490 and cell proliferation was assessed by MTS assay after 24, 48, and 72 h. AG490 inhibited cell proliferation in a dose- and time dependent manner in malignant human glioma cell lines (Fig. 1A) and non-neoplastic cell lines (Fig. 1B). The sensitivity, as assessed by the IC50, ranged from 55 to 250 μM (U87, 190 μM; T98G, 55 μM ; A172, 76 μM ; LN18, 240 μM ; LNZ308, 130 μM ; LNZ428, 120 μM) for the 72 h treatment intervals. AG490 appeared to have more potent effects on the growth of glioma cell lines than non neoplastic cell lines. Among the glioma lines, T98G seemed to be most sensitive to AG490.
Fig. 1. Effect of AG490 on cellular proliferation.
Logarithmically growing cell lines were incubated with varying concentrations of AG490 for 3 days. The relationship between AG490 and cell numbers was assessed semiquantitatively by spectrophotometric measurement of MTS bioreduction in six established (U87, A172, T98G, LN18, LNZ308, and LNZ428) malignant human glioma (A) and three non-neoplastic (B) cell lines (HAC, human cerebellar astrocyes; HA, human astrocytes; HF, human fibroblast). Control cells treated with equivalent concentrations of vehicle (DMSO). Points represent the mean of three experiments ± standard deviation. AG490 inhibited cell proliferation in a dose- and time-dependent manner in malignant human glioma cell lines. AG490 seemed to have more effect on the glioma cells than the non neoplastic cell lines. Among the glioma lines T98G was most sensitive to AG490 with an IC50 value of 55μM.
3.2. Combinatorial Efficacy of AG490 and UCN-01 in Glioma Cell Lines is Associated with p53 Function
Studies from our laboratory [24] and others [10, 11, 16, 25, 26] have shown that UCN-01 enhances the efficacy of conventional chemotherapeutic agents by exhibiting synergistic anti-proliferative and cytotoxic activity in vitro and in vivo. It has been suggested that the abrogation of G2 and/or S phase checkpoint by UCN-01 preferentially sensitizes p53 defective cells, in which the G1/S checkpoint is already compromised. To evaluate the generalizability of the potential synergy between AG490 and UCN01 in glioma cell lines, and the possible contribution of p53 function to the potentiation of UCN-01 cytotoxicity by AG490, we tested the combined effect of UCN-01 with AG490 in U87, A172 (p53 wild type), T98G (p53 defective) and LNZ308 (p53 deleted) cell lines. Cells were incubated with varying concentrations of UCN-01 and 10 μM AG490, a concentration well below IC50 in human glioma cell lines (see Fig. 2A), and then cell proliferation was measured using an MTS assay. As shown in Fig. 2A, UCN-01 demonstrated inhibition of proliferation at nanomolar concentrations in each of the glioma cell lines tested. However, there was a dramatic potentiation of UCN-01 induced cytotoxicity by AG490 in p53 mutant/deleted cell lines (e.g., T98G and LNZ308). In contrast, in p53 wild type cells (e.g., U87 and A172) when the cell proliferative activity of the drug combination was determined by MTS assay, an antagonistic effect was observed (Fig. 2A).
Fig. 2. Combination of AG490 and UCN-01 preferentially inhibits p53 defective cell lines.
A. Logarithmically growing glioma cell lines were incubated with or without varying concentrations of UCN-01(nM) with or without 10 μM of AG490 for 3 days. The relationship between UCN-01 concentration and cell numbers was assessed semiquantitatively by spectrophotometric measurement of MTS bioreduction in A172 (p53 wild type), T98G (p53 defective) and LNZ308 (p53 deleted), established malignant human glioma cell lines. B. Logarithmically growing glioma cell lines were incubated with indicated concentration of UCN-01 with or without AG490 (15 μM) for 24 hrs. Apoptosis was determined by flow cytometry using Annexin V- Alexa Flour 488 as described under “Materials and Methods” following manufacturer's protocol. Points represent the mean of three experiments ± standard deviation. AG490 significantly reduced apoptosis induced by UCN-01 in p53 wild type cells (e.g., U87 and A172), whereas UCN-01-induced cytotoxicity was further potentiated by AG490 in p53 defective cell lines (e.g., LNZ308 and T98G).
To further characterize the potential interactions between AG490 and UCN-01 on cell viability, U87, A172, LNZ308, and T98G cells were exposed varying concentrations of UCN-01 with or without 15 μM of AG490 and cell viability was assessed after 24 hrs. The result (Fig. 2B) shows that AG490 significantly reduced apoptosis induced by UCN-01 in p53 wild type cells (e.g., U87 and A172), whereas UCN-01-induced cytotoxicity was further potentiated by AG490 in p53 defective cell lines (e.g., LNZ308 and T98G).
To determine whether the interaction between UCN-01 and AG490 was due to additive or synergistic interactions, we performed concentration-effect and isobologram analyses. Glioma cells were exposed to UCN-01 or AG490 either alone or in combination over a wide range of doses but at a fixed dose ratio (0.1: 10 molar ratio) for 72 h. The data were then applied to determine the combination index (CI) which provides a semiquantitative assessment of the presence of additive, synergistic or antagonistic interactions at different effect levels [23]. The combination index is 1 for additive interactions, greater than 1 for antagonistic interactions, and less than 1 for synergistic interactions. The combination of UCN-01 and AG490 produced a synergistic inhibition in p53 mutant cell lines, based on the observation that the CI was substantially less than 1, whereas an antagonistic effect was observed in p53 wild type cell lines (data not shown). These results suggest that the potentiation of UCN-01 cytotoxicity by AG490 was selectively manifested in cells with defective p53 function, which resembles the results observed in other tumor types with combination of UCN-01 with cis-diamminedichloroplatinum (CDDP), camptothecin, mitomycin C, and irradiation [10, 11, 16, 27].
3.3. Effect of AG490 and UCN-01 on Cell Cycle Progression and Cell Cycle Regulatory Proteins
To better understand the p53-dependent basis for the synergistic inhibition of cell growth by AG490 and UCN-01, we studied the effect of these inhibitors alone or in combination on A172 (p53 wild type) and T98G (p53 mutant) cell lines. Cell cycle progression was evaluated via flow cytometry. The effect of AG490 and UCN-01 treatment on cell cycle phase distribution in A172 and T98G cell lines is summarized in Table 1. When cells were exposed to UCN-01, a distinct G1 cell cycle block with a concomitant loss of those cells in S and G2/M phase is demonstrated. AG490 alone had no significant effect on cell cycle progression in A172 cells but induced a G2/M arrest in T98G cells. Combined exposure to AG490 and UCN-01 resulted in a dramatic decrease in G2/M fraction and induced a substantial sub-G1 (apoptotic) fraction in T98G cells.
We then studied the effect of AG490 and UCN-01 alone or in combination on the expression level of various cell cycle regulatory proteins. UCN-01 or AG490 or the combination of both had very little effect on the expression level of cyclin D1, cyclin D3, CDK4 and CDK6 in A172, T98G and LNZ308 cells (Fig. 3).
Fig. 3. Combination of AG490 and UCN-01 on cell cycle regulatory proteins.
Logarithmically growing A172, T98G, and LNZ308 cells were incubated for 3 days in the presence of 25 μM AG490 with or without UCN-01 (200 nM). The cells were lysed, and proteins were separated by SDS-PAGE and probed with specific antibodies against cyclin D1, or cyclin D3, or cyclin dependent kinase (CDK) 4, or CDK 6. Western analysis was performed as described in “Materials and Methods”. The blots were subsequently stripped and reprobed with antibodies against β-actin. UCN-01 or AG490 or the combination of both had very little effect on the expression level of cell cycle regulatory proteins cyclin D1, cyclin D3, CDK4 and CDK6 in A172, T98G and LNZ308 cells.
3.4. Combination of AG490 and UCN-01 Induces p18 BAX and Cleaved PARP Expression in T98G cells
Drug-induced apoptosis is associated with characteristic morphological changes accompanied by activation of one or more proteins that trigger apoptotic signaling. BAX has been shown to undergo post-translational modification during apoptosis. For example, p18 BAX generation through wild type BAX cleavage has been observed in response to various chemotherapeutic agents [28]. To evaluate the apoptotic mechanism, we examined the level of cleaved BAX, and PARP. Cells were treated with AG490 and UCN-01 or the combination of both for varying durations and the apoptotic cleavage of BAX and PARP was assessed using specific antibodies that recognize the respective cleaved products by Western immunoblotting. In both T98G and A172 cells, treatment with AG490 or UCN-01 alone had very little effect on the levels of cleaved BAX (Fig. 4A), or PARP (Fig. 4B). In contrast, combined treatment with both agents resulted in a significant increase in the cleaved forms of BAX, and PARP in T98G cells (Fig. 4A, B), but no such effect in the A172 cells.
Fig. 4. Combination of AG490 and UCN-01 induces the expression p18 BAX and cleaved PARP in T98G cell lines.
Logarithmically growing A172, and T98G cells were incubated for the indicated duration in the presence of 25 μM AG490 with or without UCN-01 (200 nM). The cells were lysed, and proteins were separated by SDS-PAGE and probed with BAX antibody (A) which recognize both the wild type (p21) and cleaved product of BAX (p18) or cleaved PARP antibody (B) which recognizes 89 kDa fragment. Western analysis was performed as described in “Materials and Methods”. In both T98G and A172 cells, treatment with AG490 or UCN-01 alone had very little effect on the levels of cleaved BAX (A), or PARP(B). In contrast, combined treatment with both agents resulted in a significant increase in the cleaved forms of BAX, and PARP in T98G cells, but no such effect in the A172 cells.
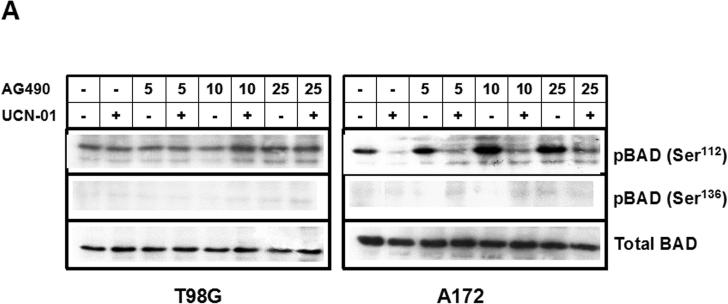
3.5. AG490 Induces BAD Phosphorylation in A172 but not in T98G cell line
In parallel with the above analyses of pro-apoptotic Bcl family members, we also examined the effect of UCN-01 and AG490 on various anti-apoptotic Bcl family members. In particular, phosphorylation of BAD has been noted to inhibit apoptotic signaling. Although BAD phosphorylation at Ser112, one of the sites critical for BAD function [29], was unaffected by either UCN-01 or AG490 in the T98G cell line, opposing effects were observed in A172 (Fig. 5A). Whereas UCN-01 decreased BAD phosphorylation in this cell line, AG490 functioned to increase p-BAD, an effect that was only partially reversed by administration of UCN-01.
Fig. 5. AG490 Induces BAD Phosphorylation at Ser112 in A172 but not in T98G cell line.
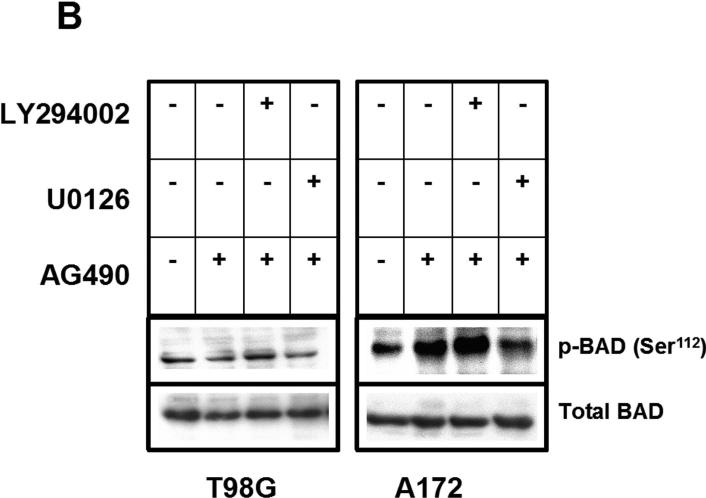
(A) Logarithmically growing A172, and T98G cells were incubated with varying concentrations of AG490 (μM) with or without 200 nM UCN-01 for 24 h. The cells were lysed, and proteins were separated by SDS-PAGE and probed with the indicated antibodies. Phosphorylation of BAD has been noted to inhibit apoptotic signaling. BAD phosphorylation at Ser112, critical for BAD function, was unaffected by either UCN-01 or AG490 in the T98G cell line. Opposing effects were observed in A172. There is no phosphorylation of BAD at Ser136 either in A172 or T98G cells. (B). Logarithmically growing T98G and A172 cells were pretreated with either LY294002 (25 μM - a PI3K/Akt specific inhibitor) or with U0126 (25 μM- a MEK-specific inhibitor) 2 h prior to AG490 (25 μM) treatment for 24 h. Equal amounts of proteins were separated by SDS-PAGE and probed with p-BAD (Ser112) antibody. The blots were subsequently stripped and reprobed with antibodies against total BAD. U0126 potently blocked AG490-induced Ser112 phosphorylation of BAD in A172 cells, no effect was observed with administration of LY294002. This observation suggests that AG490 may trigger a MAPK cascade to phosphorylate and inactivate BAD in p53 wild type but not in mutants.
These observations suggest that rather than potentiating apoptotic signaling in A172, the effects of UCN-01 are in part neutralized by the survival pathways activated by AG490. Because phosphorylation of BAD at Ser112can result from activation of cell survival signaling by the Ras-MAPK signaling pathway or via Akt-mediated signaling [30, 31], we examined the effect of blocking these pathways on AG490-induced BAD phosphorylation. A172 and T98G cells were incubated with AG490 in the presence or absence of U0126 (an MEK-specific inhibitor), or LY294002 (a PI3K/Akt specific inhibitor). Cell homogenates were collected, and levels of phosphorylated BAD and total BAD levels were measured by Western blot analysis (Fig. 5B). Whereas U0126 potently blocked AG490-induced Ser112 phosphorylation of BAD in A172 cells, no effect was observed with administration of LY294002. This observation suggests that AG490 may trigger a MAPK cascade to phosphorylate and inactivate BAD in p53 wild type but not in defective cell lines.
4. Discussion
The p53 gene is inactivated in the many human cancers, resulting in profound effects on cell survival and apoptosis [32]. Much effort has therefore gone into determining the effects of p53 inactivation on the response of cancer cells to therapeutic agents. Because genetic alterations are in large part responsible for the generation and biologic properties of tumors, it is reasonable to expect that the specific alterations in tumors determine their responses to therapeutic agents. Many studies have examined the role of p53 in therapeutic responses, but the results have varied considerably. While some clinical studies have suggested an association between p53 status and therapeutic response to certain agents [33, 34], confirming such correlations is often confounded by tumor heterogeneity and technical difficulties in reliably assessing p53 inactivation in naturally occurring tumors [35].
In a previous study, we [24] have found that p53 function can influence the cell proliferation and clonogenic response of glioma cells to UCN-01. In this study, we examined the effect on cell proliferation and apoptosis of AG490 in combination with UCN-01 in glioma cell lines that have wild type or defective p53 function, and investigated the ability of AG490 to potentiate UCN-01-induced cytotoxicity. The sensitivity of cells to UCN-01 treatment is in part determined by their ability to abrogate S and G2/M checkpoint phases of the cell cycle. At sub-lethal doses, UCN-01 causes an accumulation of cells in the G1 phase, irrespective of p53 status. AG490 alone has no significant effect on cell cycle progression in A172 (p53 wild type) cells, but induces a G2/M arrest in T98G (p53 defective) cells. We have also demonstrated that AG490 triggers BAD phosphorylation at Ser112, thus preventing UCN-01-induced cell death and promoting cell survival in p53 wild type glioma cell lines.
These observations suggest that, contrary to effects of cytotoxic chemotherapy and irradiation, certain signaling-targeted therapies may actually be more efficacious in tumor cell lines with p53 gene inactivation than those with intact p53 function. It remains uncertain mechanistically why AG490 promotes BAD phosphorylation in p53 wild-type glioma cell lines, and whether other anti-apoptotic factors may also be involved in protecting against UCN-01-induced cytotoxicity. Our results suggest that phosphorylation of BAD, a Bcl-2 family protein, may represent an important bridge between survival signaling by growth factor receptors and the prevention of apoptosis. Phosphorylation of BAD at Ser112, Ser136, and Ser155 has been demonstrated to inactivate its proapoptotic function [36] by a mechanism involving binding to 14−3−3 scaffold proteins that results in sequestering BAD from mitochondria and dissociation of BAD from mitochondrial Bcl2 and/or Bcl-XL [37]. It is been observed that the pro-survival effects of the PI3K/Akt pathway are mediated through inactivation of BAD by phosphorylation at Ser136 [38], whereas MAPK-activated p90 ribosomal S6 kinase (p90RSK)-stimulated survival signaling is mediated by phosphorylation of BAD at Ser112; conversely, p90RSK has been reported to protect cells from BAD-induced apoptosis [30, 31]. In addition to its activation by the MAPK pathway, p90RSK is also activated by PKC, one of the putative targets of UCN-01 [39]. Recently, Bertolotto et al [40] have shown that over expression of PKCε, PKCα, and PKCθ in HEK 293 cells led to phosphorylation of BAD at Ser112 but not Ser136. Moreover, in cells overexpressing dominant-negative PKC isoforms, phosphorylation of BAD at Ser112 was significantly reduced. Since PKC has been reported to activate the MAPK cascade at several levels including Ras and Raf [41], and MAPK can directly phosphorylate p90RSK at Thr562 in vitro and in vivo [42, 43], it seems plausible to assume that PKC regulates p90RSK via the MAPK cascade.
The results presented here demonstrate that AG490 induces the phosphorylation of BAD at Ser112 in A172 cells, which is diminished by inhibition of the MAPK signaling pathway by U0126. BAD phosphorylation was only partially reversed by UCN-01, a result that was reflected in the protection against cytotoxicity by the combination of AG490 with UCN-01. It remains uncertain why induction of BAD phosphorylation by AG490 was not observed in p53-mutated cells and why a more pro-apoptotic response was induced by the combination of UCN-01 and AG490 in these cells.
Several mechanisms have been proposed to explain how BAD and other BH3 proteins facilitate cell death by inhibiting anti-apoptotic Bcl-2 proteins, by activating pro-death BAX and BAK, or regulating mitochondrial structure. We observed that the combination of AG490 and UCN-01 upregulated the expression of p18 BAX (cleaved fragment of BAX) in the T98G cell line. This enhanced level of p18 BAX might have been generated through cleavage of full-length BAX during apoptosis [28] to generate a more potent inducer of apoptotic cell death than full-length BAX [44].
The data presented here have several important implications for understanding and evaluating the treatment of human malignant glioma with therapeutic agents. Our work showed that AG490 can enhance UCN-01-induced cytotoxicity in p53-deleted or mutated human malignant glioma cells. Conversely, antagonistic effects on cytotoxicity were observed in glioma cells with wild-type p53. Although the mechanism for these divergent effects remains conjectural, apart from their association with opposing changes in BAD phosphorylation and BAX cleavage, the magnitude of the differences calls attention for the need to consider genotypic features in interpreting response profiles to signaling-targeted therapies for these tumors. It is important to emphasize that analyses using established tumor cell lines may have limitations as exact models of human cancers, which necessitates some caution in direct application of in vitro observations to the clinic and should not constitute a basis for bypassing evaluation of potentially promising agents. Rather, these preclinical findings may provide insights into genotypic correlates that may influence treatment response, which can help to refine clinical trial design and evaluation.
Acknowledgements
We thank Beth Arnold and Naomi Agostino for technical assistance.
Grant support: This work was supported by NIH grant P01NS40923 and a grant from the Wichman Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fine HA. The basis for current treatment recommendations for malignant gliomas. J Neurooncol. 1994;20:111–120. doi: 10.1007/BF01052722. [DOI] [PubMed] [Google Scholar]
- 2.Levin VA, Silver P, Hannigan J, Wara WM, Gutin PH, Davis RL, Wilson CB. Superiority of post-radiotherapy adjuvant chemotherapy with CCNU, procarbazine, and vincristine (PCV) over BCNU for anaplastic gliomas: NCOG 6G61 final report. Int J Radiat Oncol Biol Phys. 1990;18:321–324. doi: 10.1016/0360-3016(90)90096-3. [DOI] [PubMed] [Google Scholar]
- 3.Hartwell LH, Kastan MB. Cell cycle control and cancer. Science. 1994;266:1821–1828. doi: 10.1126/science.7997877. [DOI] [PubMed] [Google Scholar]
- 4.Nieder C, Petersen S, Petersen C, Thames HD. The challenge of p53 as prognostic and predictive factor in gliomas. Cancer Treat Rev. 2000;26:67–73. doi: 10.1053/ctrv.1999.0145. [DOI] [PubMed] [Google Scholar]
- 5.Fueyo J, Gomez-Manzano C, Yung WK, Kyritsis AP. The functional role of tumor suppressor genes in gliomas: clues for future therapeutic strategies. Neurology. 1998;51:1250–1255. doi: 10.1212/wnl.51.5.1250. [DOI] [PubMed] [Google Scholar]
- 6.Ng CE, Banerjee SK, Pavliv M, Wang G, Raaphorst GP, Aubin RA. p53 status, cellular recovery and cell cycle arrest as prognosticators of in vitro radiosensitivity in human pancreatic adenocarcinoma cell lines. Int J Radiat Biol. 1999;75:1365–1376. doi: 10.1080/095530099139241. [DOI] [PubMed] [Google Scholar]
- 7.Pollack IF, Finkelstein SD, Woods J, Burnham J, Holmes EJ, Hamilton RL, Yates AJ, Boyett JM, Finlay JL, Sposto R. Expression of p53 and prognosis in children with malignant gliomas. N Engl J Med. 2002;346:420–427. doi: 10.1056/NEJMoa012224. [DOI] [PubMed] [Google Scholar]
- 8.Lau CC, Pardee AB. Mechanism by which caffeine potentiates lethality of nitrogen mustard. Proc Natl Acad Sci U S A. 1982;79:2942–2946. doi: 10.1073/pnas.79.9.2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sarkaria JN, Busby EC, Tibbetts RS, Roos P, Taya Y, Karnitz LM, Abraham RT. Inhibition of ATM and ATR kinase activities by the radiosensitizing agent, caffeine. Cancer Res. 1999;59:4375–4382. [PubMed] [Google Scholar]
- 10.Bunch RT, Eastman A. 7-Hydroxystaurosporine (UCN-01) causes redistribution of proliferating cell nuclear antigen and abrogates cisplatin-induced S-phase arrest in Chinese hamster ovary cells. Cell Growth Differ. 1997;8:779–788. [PubMed] [Google Scholar]
- 11.Shao RG, Cao CX, Shimizu T, O'Connor PM, Kohn KW, Pommier Y. Abrogation of an S-phase checkpoint and potentiation of camptothecin cytotoxicity by 7-hydroxystaurosporine (UCN-01) in human cancer cell lines, possibly influenced by p53 function. Cancer Res. 1997;57:4029–4035. [PubMed] [Google Scholar]
- 12.Yu L, Orlandi L, Wang P, Orr MS, Senderowicz AM, Sausville EA, Silvestrini R, Watanabe N, Piwnica-Worms H, O'Connor PM. UCN-01 abrogates G2 arrest through a Cdc2-dependent pathway that is associated with inactivation of the Wee1Hu kinase and activation of the Cdc25C phosphatase. J Biol Chem. 1998;273:33455–33464. doi: 10.1074/jbc.273.50.33455. [DOI] [PubMed] [Google Scholar]
- 13.Busby EC, Leistritz DF, Abraham RT, Karnitz LM, Sarkaria JN. The radiosensitizing agent 7-hydroxystaurosporine (UCN-01) inhibits the DNA damage checkpoint kinase hChk1. Cancer Res. 2000;60:2108–2112. [PubMed] [Google Scholar]
- 14.Bunch RT, Eastman A. Enhancement of cisplatin-induced cytotoxicity by 7-hydroxystaurosporine (UCN-01), a new G2-checkpoint inhibitor. Clin Cancer Res. 1996;2:791–797. [PubMed] [Google Scholar]
- 15.Hirose Y, Berger MS, Pieper RO. Abrogation of the Chk1-mediated G(2) checkpoint pathway potentiates temozolomide-induced toxicity in a p53-independent manner in human glioblastoma cells. Cancer Res. 2001;61:5843–5849. [PubMed] [Google Scholar]
- 16.Wang Q, Fan S, Eastman A, Worland PJ, Sausville EA, O'Connor PM. UCN-01: a potent abrogator of G2 checkpoint function in cancer cells with disrupted p53. J Natl Cancer Inst. 1996;88:956–965. doi: 10.1093/jnci/88.14.956. [DOI] [PubMed] [Google Scholar]
- 17.Bowman T, Garcia R, Turkson J, Jove R. STATs in oncogenesis. Oncogene. 2000;19:2474–2488. doi: 10.1038/sj.onc.1203527. [DOI] [PubMed] [Google Scholar]
- 18.Leaman DW, Leung S, Li X, Stark GR. Regulation of STAT-dependent pathways by growth factors and cytokines. Faseb J. 1996;10:1578–1588. [PubMed] [Google Scholar]
- 19.Takemoto S, Mulloy JC, Cereseto A, Migone TS, Patel BK, Matsuoka M, Yamaguchi K, Takatsuki K, Kamihira S, White JD, Leonard WJ, Waldmann T, Franchini G. Proliferation of adult T cell leukemia/lymphoma cells is associated with the constitutive activation of JAK/STAT proteins. Proc Natl Acad Sci U S A. 1997;94:13897–13902. doi: 10.1073/pnas.94.25.13897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weber-Nordt RM, Mertelsmann R, Finke J. The JAK-STAT pathway: signal transduction involved in proliferation, differentiation and transformation. Leuk Lymphoma. 1998;28:459–467. doi: 10.3109/10428199809058353. [DOI] [PubMed] [Google Scholar]
- 21.Van Meir EG, Kikuchi T, Tada M, Li H, Diserens AC, Wojcik BE, Huang HJ, Friedmann T, de Tribolet N, Cavenee WK. Analysis of the p53 gene and its expression in human glioblastoma cells. Cancer Res. 1994;54:649–652. [PubMed] [Google Scholar]
- 22.Riss TL, Moravec RA. Use of multiple assay endpoints to investigate the effects of incubation time, dose of toxin, and plating density in cell-based cytotoxicity assays. Assay Drug Dev Technol. 2004;2:51–62. doi: 10.1089/154065804322966315. [DOI] [PubMed] [Google Scholar]
- 23.Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 24.Pollack IF, Kawecki S, Lazo JS. Blocking of glioma proliferation in vitro and in vivo and potentiating the effects of BCNU and cisplatin: UCN-01, a selective protein kinase C inhibitor. J Neurosurg. 1996;84:1024–1032. doi: 10.3171/jns.1996.84.6.1024. [DOI] [PubMed] [Google Scholar]
- 25.Akinaga S, Nomura K, Gomi K, Okabe M. Enhancement of antitumor activity of mitomycin C in vitro and in vivo by UCN-01, a selective inhibitor of protein kinase C. Cancer Chemother Pharmacol. 1993;32:183–189. doi: 10.1007/BF00685833. [DOI] [PubMed] [Google Scholar]
- 26.Monks A, Harris ED, Vaigro-Wolff A, Hose CD, Connelly JW, Sausville EA. UCN-01 enhances the in vitro toxicity of clinical agents in human tumor cell lines. Invest New Drugs. 2000;18:95–107. doi: 10.1023/a:1006313611677. [DOI] [PubMed] [Google Scholar]
- 27.Sugiyama K, Shimizu M, Akiyama T, Tamaoki T, Yamaguchi K, Takahashi R, Eastman A, Akinaga S. UCN-01 selectively enhances mitomycin C cytotoxicity in p53 defective cells which is mediated through S and/or G(2) checkpoint abrogation. Int J Cancer. 2000;85:703–709. doi: 10.1002/(sici)1097-0215(20000301)85:5<703::aid-ijc17>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 28.Wood DE, Thomas A, Devi LA, Berman Y, Beavis RC, Reed JC, Newcomb EW. Bax cleavage is mediated by calpain during drug-induced apoptosis. Oncogene. 1998;17:1069–1078. doi: 10.1038/sj.onc.1202034. [DOI] [PubMed] [Google Scholar]
- 29.Zha J, Harada H, Yang E, Jockel J, Korsmeyer SJ. Serine phosphorylation of death agonist BAD in response to survival factor results in binding to 14−3−3 not BCL-X(L) Cell. 1996;87:619–628. doi: 10.1016/s0092-8674(00)81382-3. [DOI] [PubMed] [Google Scholar]
- 30.Blenis J. Signal transduction via the MAP kinases: proceed at your own RSK. Proc Natl Acad Sci U S A. 1993;90:5889–5892. doi: 10.1073/pnas.90.13.5889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bonni A, Brunet A, West AE, Datta SR, Takasu MA, Greenberg ME. Cell survival promoted by the Ras-MAPK signaling pathway by transcription-dependent and -independent mechanisms. Science. 1999;286:1358–1362. doi: 10.1126/science.286.5443.1358. [DOI] [PubMed] [Google Scholar]
- 32.Bunz F, Hwang PM, Torrance C, Waldman T, Zhang Y, Dillehay L, Williams J, Lengauer C, Kinzler KW, Vogelstein B. Disruption of p53 in human cancer cells alters the responses to therapeutic agents. J Clin Invest. 1999;104:263–269. doi: 10.1172/JCI6863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Benhattar J, Cerottini JP, Saraga E, Metthez G, Givel JC. p53 mutations as a possible predictor of response to chemotherapy in metastatic colorectal carcinomas. Int J Cancer. 1996;69:190–192. doi: 10.1002/(SICI)1097-0215(19960621)69:3<190::AID-IJC7>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 34.Brett MC, Pickard M, Green B, Howel-Evans A, Smith D, Kinsella A, Poston G. p53 protein overexpression and response to biomodulated 5-fluorouracil chemotherapy in patients with advanced colorectal cancer. Eur J Surg Oncol. 1996;22:182–185. doi: 10.1016/s0748-7983(96)90827-6. [DOI] [PubMed] [Google Scholar]
- 35.Hall PA, Lane DP. p53 in tumour pathology: can we trust immunohistochemistry?--Revisited! J Pathol. 1994;172:1–4. doi: 10.1002/path.1711720103. [DOI] [PubMed] [Google Scholar]
- 36.Tan Y, Demeter MR, Ruan H, Comb MJ. BAD Ser-155 phosphorylation regulates BAD/Bcl-XL interaction and cell survival. J Biol Chem. 2000;275:25865–25869. doi: 10.1074/jbc.M004199200. [DOI] [PubMed] [Google Scholar]
- 37.Datta SR, Katsov A, Hu L, Petros A, Fesik SW, Yaffe MB, Greenberg ME. 14−3−3 proteins and survival kinases cooperate to inactivate BAD by BH3 domain phosphorylation. Mol Cell. 2000;6:41–51. [PubMed] [Google Scholar]
- 38.Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg ME. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91:231–241. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- 39.Fisher TL, Blenis J. Evidence for two catalytically active kinase domains in pp90rsk. Mol Cell Biol. 1996;16:1212–1219. doi: 10.1128/mcb.16.3.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bertolotto C, Maulon L, Filippa N, Baier G, Auberger P. Protein kinase C theta and epsilon promote T-cell survival by a rsk-dependent phosphorylation and inactivation of BAD. J Biol Chem. 2000;275:37246–37250. doi: 10.1074/jbc.M007732200. [DOI] [PubMed] [Google Scholar]
- 41.Marshall MS. Ras target proteins in eukaryotic cells. Faseb J. 1995;9:1311–1318. doi: 10.1096/fasebj.9.13.7557021. [DOI] [PubMed] [Google Scholar]
- 42.Dalby KN, Morrice N, Caudwell FB, Avruch J, Cohen P. Identification of regulatory phosphorylation sites in mitogen-activated protein kinase (MAPK)-activated protein kinase-1a/p90rsk that are inducible by MAPK. J Biol Chem. 1998;273:1496–1505. doi: 10.1074/jbc.273.3.1496. [DOI] [PubMed] [Google Scholar]
- 43.Grove JR, Price DJ, Banerjee P, Balasubramanyam A, Ahmad MF, Avruch J. Regulation of an epitope-tagged recombinant Rsk-1 S6 kinase by phorbol ester and erk/MAP kinase. Biochemistry. 1993;32:7727–7738. doi: 10.1021/bi00081a018. [DOI] [PubMed] [Google Scholar]
- 44.Toyota H, Yanase N, Yoshimoto T, Moriyama M, Sudo T, Mizuguchi J. Calpain-induced Bax-cleavage product is a more potent inducer of apoptotic cell death than wild-type Bax. Cancer Lett. 2003;189:221–230. doi: 10.1016/s0304-3835(02)00552-9. [DOI] [PubMed] [Google Scholar]