Abstract
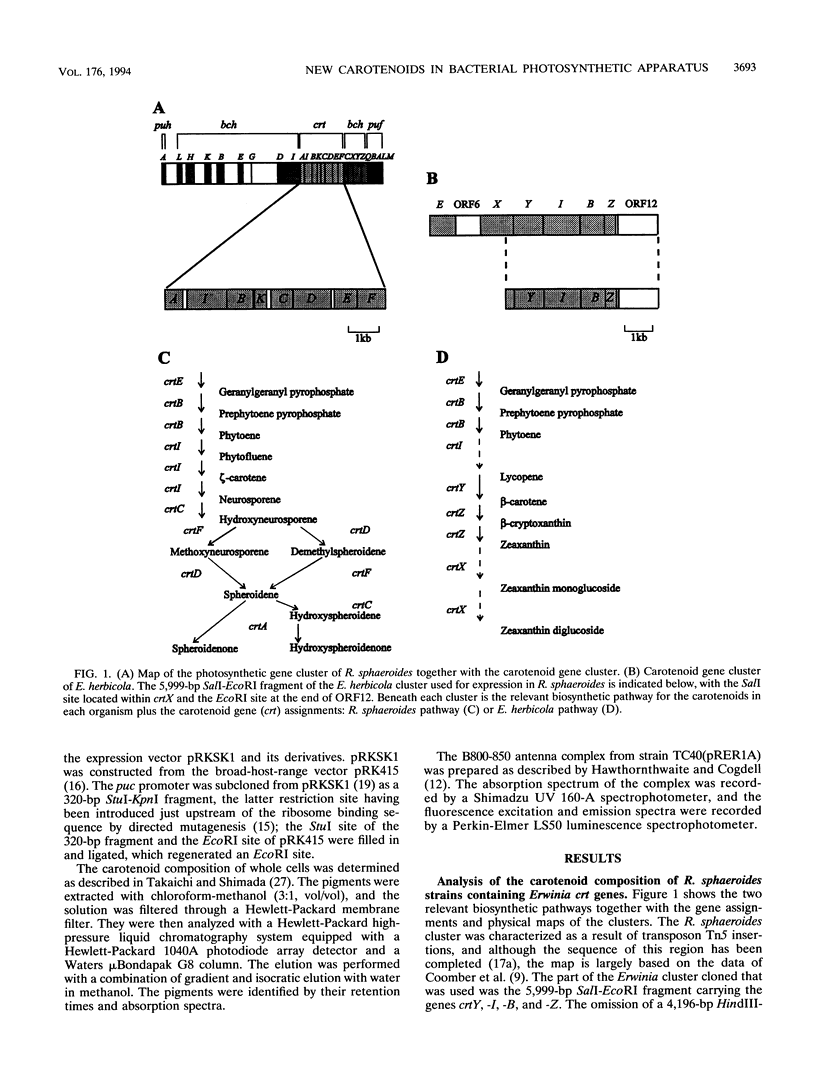
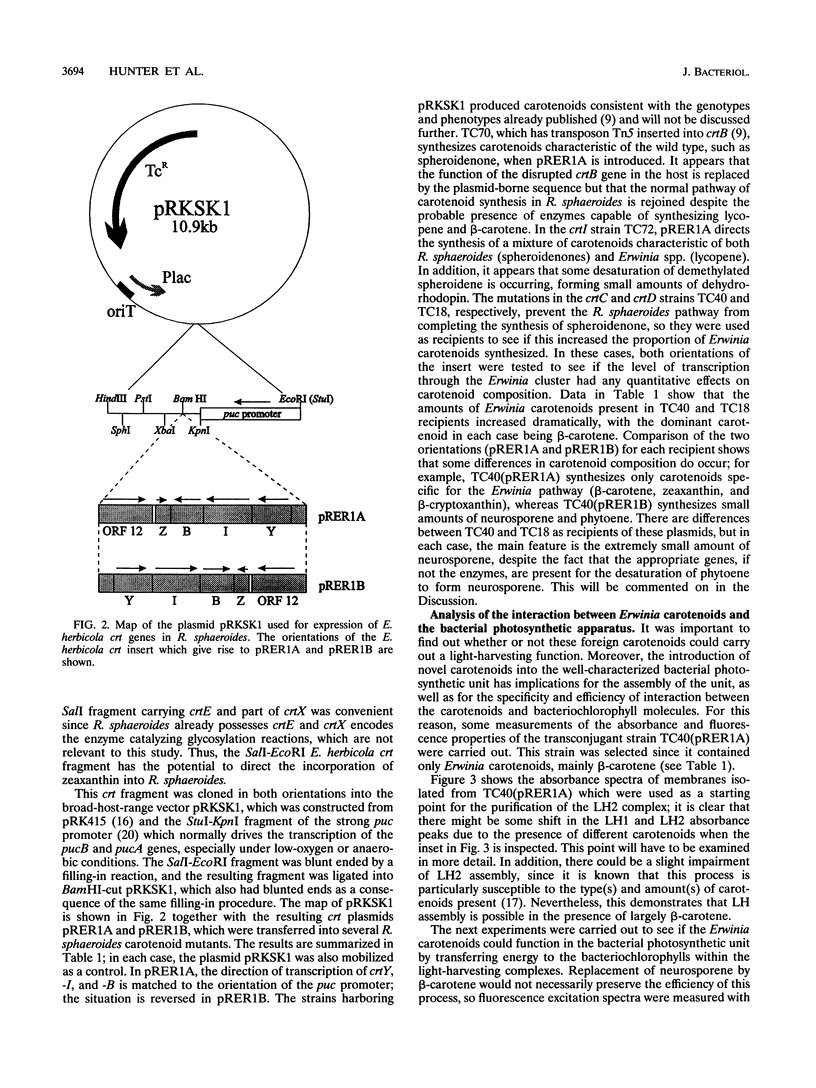
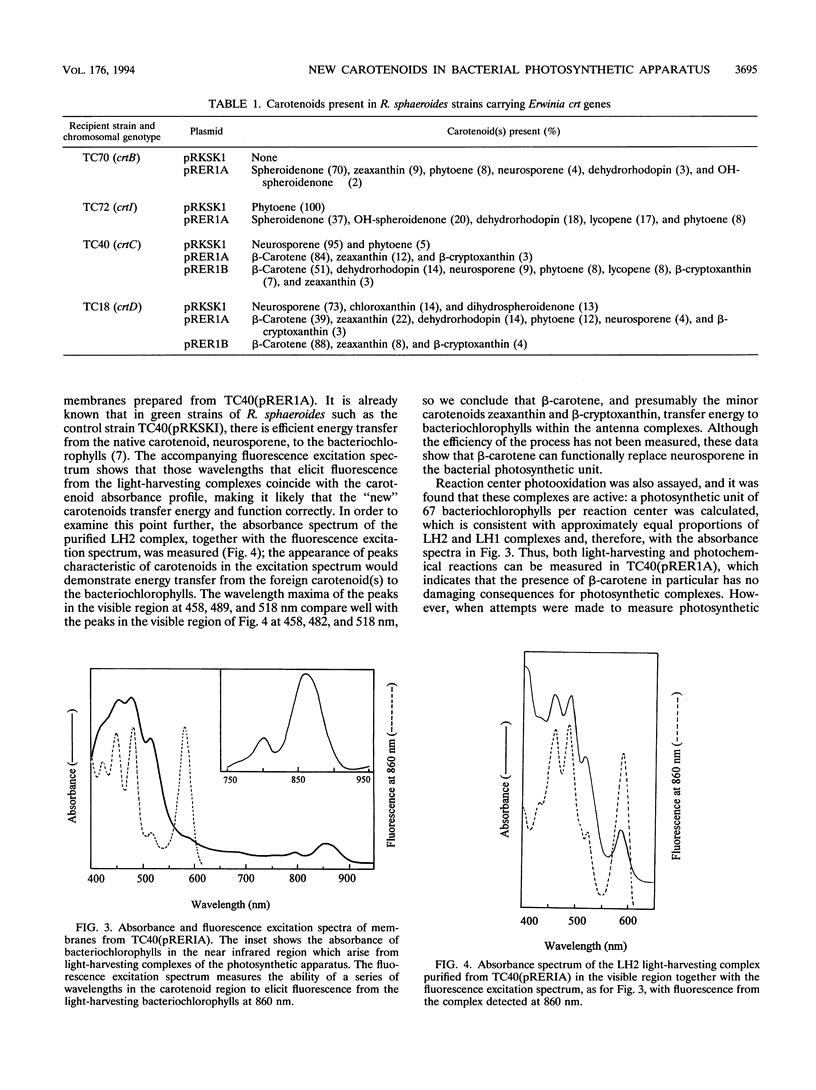
Carotenoids have two major functions in bacterial photosynthesis, photoprotection and accessory light harvesting. The genes encoding many carotenoid biosynthetic pathways have now been mapped and cloned in several different species, and the availability of cloned genes which encode the biosynthesis of carotenoids not found in the photosynthetic genus Rhodobacter opens up the possibility of introducing a wider range of foreign carotenoids into the bacterial photosynthetic apparatus than would normally be available by producing mutants of the native biosynthetic pathway. For example, the crt genes from Erwinia herbicola, a gram-negative nonphotosynthetic bacterium which produces carotenoids in the sequence of phytoene, lycopene, beta-carotene, beta-cryptoxanthin, zeaxanthin, and zeaxanthin glucosides, are clustered within a 12.8-kb region and have been mapped and partially sequenced. In this paper, part of the E. herbicola crt cluster has been excised and expressed in various crt strains of Rhodobacter sphaeroides. This has produced light-harvesting complexes with a novel carotenoid composition, in which the foreign carotenoids such as beta-carotene function successfully in light harvesting. The outcome of the combination of the crt genes in R. sphaeroides with those from E. herbicola has, in some cases, resulted in an interesting rerouting of the expected biosynthetic sequence, which has also provided insights into how the various enzymes of the carotenoid biosynthetic pathway might interact. Clearly this approach has considerable potential for studies on the control and organization of carotenoid biosynthesis, as well as providing novel pigment-protein complexes for functional studies.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong G. A., Alberti M., Hearst J. E. Conserved enzymes mediate the early reactions of carotenoid biosynthesis in nonphotosynthetic and photosynthetic prokaryotes. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9975–9979. doi: 10.1073/pnas.87.24.9975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong G. A., Alberti M., Leach F., Hearst J. E. Nucleotide sequence, organization, and nature of the protein products of the carotenoid biosynthesis gene cluster of Rhodobacter capsulatus. Mol Gen Genet. 1989 Apr;216(2-3):254–268. doi: 10.1007/BF00334364. [DOI] [PubMed] [Google Scholar]
- CLAYTON R. K., SMITH C. Rhodopseudomonas spheroides: high catalase and blue-green double mutants. Biochem Biophys Res Commun. 1960 Aug;3:143–145. doi: 10.1016/0006-291x(60)90210-2. [DOI] [PubMed] [Google Scholar]
- Cogdell R. J., Frank H. A. How carotenoids function in photosynthetic bacteria. Biochim Biophys Acta. 1987;895(2):63–79. doi: 10.1016/s0304-4173(87)80008-3. [DOI] [PubMed] [Google Scholar]
- Cogdell R. J., Hipkins M. F., MacDonald W., Truscott T. G. Energy transfer between the carotenoid and the bacteriochlorophyll within the B-800-850 light-harvesting pigment-protein complex of Rhodopseudomonas sphaeroides. Biochim Biophys Acta. 1981 Jan 14;634(1):191–202. doi: 10.1016/0005-2728(81)90138-9. [DOI] [PubMed] [Google Scholar]
- Cogdell R. J., Monger T. G., Parson W. W. Carotenoid triplet states in reaction centers from Rhodopseudomonas sphaeroides and Rhodospirillum rubrum. Biochim Biophys Acta. 1975 Dec 11;408(3):189–199. doi: 10.1016/0005-2728(75)90122-x. [DOI] [PubMed] [Google Scholar]
- Coomber S. A., Chaudhri M., Connor A., Britton G., Hunter C. N. Localized transposon Tn5 mutagenesis of the photosynthetic gene cluster of Rhodobacter sphaeroides. Mol Microbiol. 1990 Jun;4(6):977–989. doi: 10.1111/j.1365-2958.1990.tb00670.x. [DOI] [PubMed] [Google Scholar]
- Hundle B. S., Beyer P., Kleinig H., Englert G., Hearst J. E. Carotenoids of Erwinia herbicola and an Escherichia coli HB101 strain carrying the Erwinia herbicola carotenoid gene cluster. Photochem Photobiol. 1991 Jul;54(1):89–93. doi: 10.1111/j.1751-1097.1991.tb01989.x. [DOI] [PubMed] [Google Scholar]
- Jones M. R., Fowler G. J., Gibson L. C., Grief G. G., Olsen J. D., Crielaard W., Hunter C. N. Mutants of Rhodobacter sphaeroides lacking one or more pigment-protein complexes and complementation with reaction-centre, LH1, and LH2 genes. Mol Microbiol. 1992 May;6(9):1173–1184. doi: 10.1111/j.1365-2958.1992.tb01556.x. [DOI] [PubMed] [Google Scholar]
- Keen N. T., Tamaki S., Kobayashi D., Trollinger D. Improved broad-host-range plasmids for DNA cloning in gram-negative bacteria. Gene. 1988 Oct 15;70(1):191–197. doi: 10.1016/0378-1119(88)90117-5. [DOI] [PubMed] [Google Scholar]
- Lang H. P., Hunter C. N. The relationship between carotenoid biosynthesis and the assembly of the light-harvesting LH2 complex in Rhodobacter sphaeroides. Biochem J. 1994 Feb 15;298(Pt 1):197–205. doi: 10.1042/bj2980197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Laborda A., Balsalobre J. M., Fontes M., Murillo F. J. Accumulation of carotenoids in structural and regulatory mutants of the bacterium Myxococcus xanthus. Mol Gen Genet. 1990 Sep;223(2):205–210. doi: 10.1007/BF00265055. [DOI] [PubMed] [Google Scholar]
- McGlynn P., Hunter C. N. Isolation and characterization of a putative transcription factor involved in the regulation of the Rhodobacter sphaeroides pucBA operon. J Biol Chem. 1992 Jun 5;267(16):11098–11103. [PubMed] [Google Scholar]
- Misawa N., Nakagawa M., Kobayashi K., Yamano S., Izawa Y., Nakamura K., Harashima K. Elucidation of the Erwinia uredovora carotenoid biosynthetic pathway by functional analysis of gene products expressed in Escherichia coli. J Bacteriol. 1990 Dec;172(12):6704–6712. doi: 10.1128/jb.172.12.6704-6712.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SISTROM W. R., GRIFFITHS M., STANIER R. Y. The biology of photosynthetic bacterium which lacks colored carotenoids. J Cell Physiol. 1956 Dec;48(3):473–515. doi: 10.1002/jcp.1030480309. [DOI] [PubMed] [Google Scholar]
- Sandmann G., Kowalczyk S. In vitro carotenogenesis and characterization of the phytoene desaturase reaction in Anacystis. Biochem Biophys Res Commun. 1989 Sep 15;163(2):916–921. doi: 10.1016/0006-291x(89)92309-7. [DOI] [PubMed] [Google Scholar]
- Schnurr G., Schmidt A., Sandmann G. Mapping of a carotenogenic gene cluster from Erwinia herbicola and functional identification of six genes. FEMS Microbiol Lett. 1991 Mar 1;62(2-3):157–161. doi: 10.1016/0378-1097(91)90151-y. [DOI] [PubMed] [Google Scholar]
- Scolnik P. A., Zannoni D., Marrs B. L. Spectral and functional comparisons between the carotenoids of the two antenna complexes of Rhodopseudomonas capsulata. Biochim Biophys Acta. 1980 Dec 3;593(2):230–240. doi: 10.1016/0005-2728(80)90061-4. [DOI] [PubMed] [Google Scholar]
- Zsebo K. M., Hearst J. E. Genetic-physical mapping of a photosynthetic gene cluster from R. capsulata. Cell. 1984 Jul;37(3):937–947. doi: 10.1016/0092-8674(84)90428-8. [DOI] [PubMed] [Google Scholar]


