Abstract
Synergistic multimodality therapy is needed for breast cancer. Breast cancer frequently has p53 mutations that result in cells less likely to undergo apoptosis when exposed to DNA damaging therapies. Taxol (paclitaxel) is more effective in the presence of mutant p53. 90Y-labeled DOTA-peptide-ChL6 (90Y-ChL6, where ChL6 is chimeric L6 antibody and DOTA is 1,4,7,10-tetraazacyclododecane-N,N′,N",N‴-tetraacetic acid) is a novel radioimmunoconjugate for targeting radiation to cancer. It has a stable metal chelator and a peptide linker that can be catabolized by hepatic lysozymes. This study was designed to assess potential synergism between Taxol and 90Y-ChL6 in a highly anaplastic breast cancer model, HBT 3477. There was no tumor response in mice receiving ChL6 or Taxol alone. In mice receiving 90Y-ChL6 alone, 79% (15 of 19) of tumors responded although none were cured. If Taxol was administered 24–72 hours before 90Y-ChL6, again, 79% (23 of 29) of tumors responded but 21% were cured. When Taxol was administered 6 or 24 hours after 90Y-ChL6, 100% (46 of 46) of tumors responded and 48% were cured. Taxol given with 90Y-ChL6 did not substantially increase toxicity. Enhancement of the therapeutic effect when Taxol was added to 90Y-ChL6 therapy for HBT 3477 xenografts was striking. The synergistic therapeutic effect of Taxol with 90Y-ChL6 may relate to the p53 mutant status and BCL2 expression in HBT 3477 cells, observations that increase the likelihood that the results of this study are relevant to therapy for breast cancer in patients. In conclusion, Taxol seemed to be synergistic with 90Y-ChL6 in this human breast cancer model. Up to 50% of these anaplastic breast cancer xenografts were cured by combined modality therapy.
Keywords: antibody, breast carcinoma
Novel, synergistic, multimodality therapy is needed for breast cancer to combat the molecular mechanisms, genetic mutations, and epigenetic abnormalities that protect the cancer from therapeutic interventions. Breast cancer frequently has p53 mutations and produces Bcl2 protein that result in cells less likely to undergo apoptosis when exposed to DNA damaging therapies, such as chemotherapy or radiotherapy (1–5). Hypoxia and dysregulation of cellular oncogenes further select for survival and growth of cancer cell mutants (1–4). Because metastatic breast cancer is currently incurable with standard multimodality therapy, novel strategies are necessary (6). Radioimmunotherapy, a systemic targeted radiation modality, damages tumor DNA while maintaining a high therapeutic index (7, 8). Studies in patients with advanced resistant breast cancer showed cancer responses (9, 10) that warrant combinations of this modality with other agents that enhance apoptosis.
131I-labeled ChL6 radioimmunotherapy has induced significant, albeit temporary, therapeutic responses in patients with incurable, metastatic breast cancer (9, 10). Studies in a human breast cancer xenograft model of the novel radioimmunoconjugate, 90Y-labeled DOTA-peptide-ChL6 (90Y-ChL6, where ChL6 is chimeric L6 antibody and DOTA is 1,4,7,10-tetraazacyclododecane-N,N′,N",N‴-tetraacetic acid) showed that most tumors responded to 9.6 MBq of 90Y-ChL6, but tumors were not cured (11, 12). 90Y-ChL6 has an exceptionally stable 90Y chelate and a biodegradable linker that reduce radiation to normal tissues.
The role of p53 in monitoring DNA damage is now well established, in part by the inability of cells lacking functional p53 to arrest in G1 phase after DNA damage (1, 2). G1 arrest provides time for DNA repair to maintain chromosomal fidelity and to promote survival of normal cells (1–4). Increased resistance to radiation and other DNA damaging agents has usually been observed in p53 null or mutated cells (1–4). However, both p53-dependent and p53-independent cell deaths are inhibited by BCL2 (13).
Taxol (paclitaxel) has been shown to have efficacy in ovarian and breast cancers because it stabilizes microtubule formation resulting in mitotic block, BCL2 disfunction, and activation of apoptosis (14–17). Taxol is more effective in the presence of mutant p53 (2, 14). The study reported herein was designed to determine the potential for synergistic effects of Taxol and 90Y-ChL6 on human breast cancer with mutant p53 protein expression. Because sequence, timing, and dose of these agents could be critical for synergism, a modified response surface approach was used to evaluate the therapeutic combination.
MATERIALS AND METHODS
Reagents.
Carrier-free 90Y (Pacific Northwest National Laboratory, Richland, WA) was purchased as chloride in 0.05 M HCl. Human-use-grade chimeric L6 (ChL6; Bristol–Myers Squibb Pharmaceutical Research Institute, Seattle), an antibody chimera consisting of a human IgG1 constant region and the Fab′ region of murine mAb L6 was used for the radioimmunoconjugate (18). ChL6 reacts with an integral membrane glycoprotein highly expressed on human breast, colon, ovary, and lung carcinomas (19, 20). Taxol (paclitaxel, Bristol–Myers Squibb), a natural product from the taxane group with antitumor activity and novel antimicrotubule properties, was obtained as a nonaqueous solution and diluted in 0.9% sodium chloride for injection. Mouse doses of 300 and 600 μg of Taxol were calculated to represent 37 and 75 mg/m2 in human dose equivalents, if it is assumed that the mouse is a 20-g standard mouse (21). This is below the range of doses being used for treatment of women with metastatic breast cancer (range 135–250 mg/m2) (22).
Cell Lines.
HBT 3477, a human breast adenocarcinoma cell line, was obtained from Bristol–Myers Squibb Pharmaceutical Research Institute (Seattle). HBT 3477 tumors are aneuploid, with a DNA index of 1.5, and negative for estrogen and progesterone receptors by immunohistochemistry; greater than 70% of HBT 3477 cells stained with L6 in the type 1 pattern defined by Mattes et al. (23) (Prodex, Aeron Biotechnology, San Leandro, CA). p53 has been shown to be mutant, deleting the region detecting double-stranded DNA breaks, and BCL2 expression is present (24).
90Y-ChL6.
90Y-ChL6 (90Y-labeled DOTA-peptide-ChL6) was prepared by prelabeling the DOTA (1,4,7,10-tetraazacyclododecane-N,N′,N",N‴-tetraacetic acid) chelate before generating the radioimmunoconjugate, as described (11, 25). By HPLC, TLC, and cellulose acetate electrophoresis, greater than 98% was 90Y-ChL6, and less than 4% of 90Y-ChL6 was aggregated in all preparations. Immunoreactivity of 90Y-ChL6 by cell-binding RIA using the HBT 3477 breast adenocarcinoma cell line as described (11) showed 100% binding relative to unmodified ChL6.
Mouse Studies.
Female athymic BALB/c nu/nu mice (Harlan Sprague–Dawley), 7–9 weeks of age, were maintained according to University of California animal care guidelines on a normal diet ad libitum and under pathogen-free conditions. Five mice were housed per cage. To minimize ambient radiation, bedding was changed daily for 1 week after treatment with 90Y-ChL6, and twice weekly thereafter. HBT 3477 cells grown in Iscove’s medium (GIBCO/BRL) were harvested in logarithmic phase; 2.5–5.0 × 106 cells were injected subcutaneously into both sides of the abdomen of each mouse. Studies were initiated 3 weeks after implantation, when tumors were 28–328 mm3.
Treatment groups of 5–10 mice received one dose of Taxol i.p. (300 or 600 μg) prior to (−72, −48, or −24 hours) or after (+6 or +24 hours) 9.6 MBq (260 μCi) of 90Y-ChL6 (315 μg) i.v. Control groups of tumored mice received 300 or 600 μg of Taxol, 9.6 MBq of 90Y-ChL6, 315 μg of unmodified ChL6, 315 μg of ChL6 with 600 μg of Taxol (+24 hours), or no treatment (Table 1). Survival was monitored daily; mouse weight, tumor size, and blood counts were measured two or three times per week for 12 weeks after injection or until death. Tumors were measured with calipers in three orthogonal diameters three times per week. Tumor volume was calculated as described by the formula for hemiellipsoids (26).
Table 1.
Tumor responses in HBT 3477 human breast cancer xenografts in mice
| Treatment | No. tumors/no. mice | Initial tumor volume, mm3 (range) | Mortality (<30 days) | No. tumor(s)
|
Response rate, % | ||
|---|---|---|---|---|---|---|---|
| PR | CR | C | |||||
| Control | |||||||
| No treatment | 17/10 | 82 (28–146) | 1 | 3 | 0 | 0 | 18 |
| ChL6 (315 μg) | 16/8 | 160 (55–328) | 1 | 0 | 0 | 2 | 13 |
| Taxol (600 μg) | 6/5 | 149 (55–250) | 1 | 0 | 0 | 0 | 0 |
| Taxol (300 μg) | 6/4 | 187 (55–273) | 0 | 0 | 0 | 0 | 0 |
| ChL6 (315 μg) + Taxol (600 μg; +24 h) | 12/7 | 141 (50–225) | 2 | 3 | 0 | 0 | 25 |
| 90Y-ChL6 | 19/10 | 100 (44–196) | 0 | 12 | 3 | 0 | 79 |
| 90Y-ChL6 + Taxol therapy | |||||||
| 90Y-ChL6 + Taxol (600 μg; −72 h) | 8/5 | 101 (39–182) | 0 | 2 | 0 | 1 | 38 |
| 90Y-ChL6 + Taxol (600 μg; −48 h) | 4/3 | 146 (62–218) | 1 | 2 | 0 | 1 | 75 |
| 90Y-ChL6 + Taxol (300 μg; −24 h) | 8/5 | 104 (62–164) | 0 | 5 | 1 | 2 | 100 |
| 90Y-ChL6 + Taxol (600 μg; −24 h) | 9/5 | 146 (87–218) | 0 | 7 | 0 | 2 | 100 |
| 90Y-ChL6 + Taxol (300 μg; +6 h) | 10/5 | 109 (44–187) | 0 | 3 | 2 | 5 | 100 |
| 90Y-ChL6 + Taxol (600 μg; + 6 h) | 18/10 | 112 (47–237) | 0 | 5 | 5 | 8 | 100 |
| 90Y-ChL6 + Taxol (600 μg; +24 h) | 18/10 | 90 (33–187) | 0 | 3 | 6 | 9 | 100 |
Response rate = cure (C) + complete regression (CR) + partial regression (PR). Control mice did not receive a combination of 90Y-ChL6 and Taxol. 90Y-ChL6 was always at 9.6 MBq.
Blood samples were collected from tail veins with 2-μl microcapillary pipets. Samples from mice within a dose group were pooled and diluted 1:200 in PBS (0.9% saline/10 mM sodium phosphate, pH 7.6) for red blood cell counts, diluted 1:100 in 1% ammonium oxalate for platelet counts, or diluted 1:20 in 3% (wt/vol) acetic acid for white blood cell counts (27).
Tumoricidal Effect.
Initial tumor volume was defined as the volume on the day before treatment. Mean tumor volume was calculated for each group on each day of measurement; tumors that had completely regressed were considered to have a volume of zero. Tumor responses were categorized as follows: C, cure (tumor disappeared and did not regrow by the end of the 84-day study); CR, complete regression (tumor disappeared for at least 7 days, but later regrew); PR, partial regression (tumor volume decreased by 50% or more for at least 7 days then regrew).
Statistical Analysis.
Comparisons of response among treatment groups were done by the method of Cochran–Mantel–Haensel (28). The test generates a P value that indicates whether the observed differences in response among treatment groups may be due to chance. A ranking based on the quality of the response was assigned, ordered as C, CR, PR, and no response. To minimize the possibility of declaring the therapy effective when it was not, statistical testing was done in the following order. (i) A test was done to determine whether chance alone could explain observed differences among all groups. (ii) A test was done to see whether chance alone could explain any differences among the control groups, defined as those not receiving combined 90Y-ChL6 and Taxol therapy. (iii) Comparison was made among the groups receiving different combinations of 90Y-ChL6 and Taxol therapy. On the basis of statistically significant differences in the aforementioned comparisons, more specific comparisons were then performed between selected groups.
RESULTS
Tumoricidal Effect.
Tumors in mice treated with Taxol alone (300 or 600 μg), unmodified ChL6 alone, unmodified ChL6 with Taxol, or untreated grew without interruption, whereas those treated with a combination of 90Y-ChL6 and Taxol showed a dramatic decrease in volume (Fig. 1). Groups were analyzed as described using the Cochran–Mantel–Haensel method to determine whether observed differences in response could be due to chance. Six groups (76 tumors) that did not receive combined 90Y-ChL6 and Taxol were used to assess control responses (Table 1). Only the group that received 90Y-ChL6 alone differed significantly (P = 0.001) when compared with the untreated control group; all other groups used to assess control responses were not different from the untreated control or from each other (P > 0.28). Among groups of mice receiving no 90Y-ChL6, the response rates, generally partial regressions, ranged from 0 to 25% (Table 1). In mice receiving 9.6 MBq of 90Y-ChL6 alone, 79% (15 of 19) of tumors achieved a response although none were cured (Fig. 2).
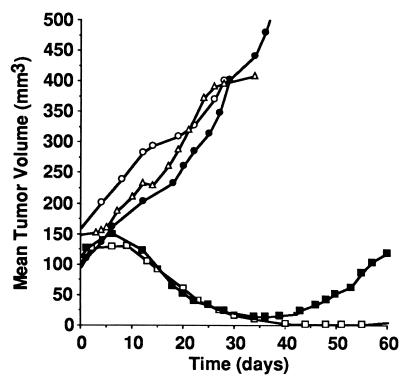
Figure 1.
Temporal course of untreated and treated HBT 3477 tumors in mice. The optimal combination of Taxol and 90Y-ChL6 (600 μg of Taxol given 24 hours after 90Y-ChL6) (□) showed dramatic tumor regression; comparable decreases were seen in all 90Y-ChL6/Taxol groups. The group of mice receiving 90Y-ChL6 alone (▪) also showed marked tumor regression but the effect was not sustained. Tumors in mice receiving 315 μg of ChL6 (○), 600 μg of Taxol (▵), or no treatment (•) grew without interruption.
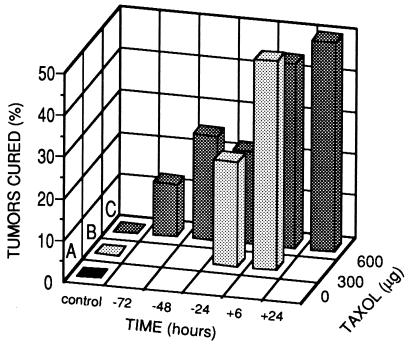
Figure 2.
Cure rate of HBT 3477 tumors in mice. Mice received one dose of Taxol (300 or 600 μg) prior to (−72, −48, or −24 hours) or after (+6 or +24 hours) 90Y-ChL6. Cure rate was much better when Taxol was given after 90Y-ChL6; there was no difference in efficacy between 300 or 600 μg of Taxol with 90Y-ChL6. No cures were observed after administration of 90Y-ChL6 alone (A), 300 μg of Taxol alone (B), or 600 μg of Taxol alone (C).
Seven groups of mice received combined 90Y-ChL6 and Taxol therapy. In mice receiving combined 90Y-ChL6 and Taxol therapy where Taxol (300 or 600 μg) was administered before 90Y-ChL6 (−72, −48, or −24 hours), 79% (23 of 29) of tumors responded and 21% (6 of 29) were cured. When Taxol (300 or 600 μg) was administered after 90Y-ChL6 (+6 or +24 hours), 100% (46 of 46) of tumors achieved a response and 48% (22 of 46) were cured (Fig. 2). The overall evaluation of these combined treatment groups showed significant differences among them (P = 0.003). Specific analysis of the five groups that received 600 μg of Taxol and 90Y-ChL6 also showed significant differences in response (P = 0.002). Further analysis of the 600-μg groups, comparing Taxol given before versus after 90Y-ChL6, revealed a significant difference (P = 0.001); mice treated with Taxol after 90Y-ChL6 had greater reduction in tumor volume than those where Taxol was given before 90Y-ChL6. There was no statistical difference between the response of tumors in mice receiving 300 μg versus 600 μg of Taxol.
Weight Loss.
Groups of mice receiving no 90Y-ChL6 showed no weight loss. Mice that received only 90Y-ChL6 lost 15% of their initial body weight but began to regain weight by day 12 after therapy. All groups receiving 90Y-ChL6 and Taxol demonstrated similar transient weight loss (3–16% of initial body weight) and subsequent weight gain.
Myelotoxicity.
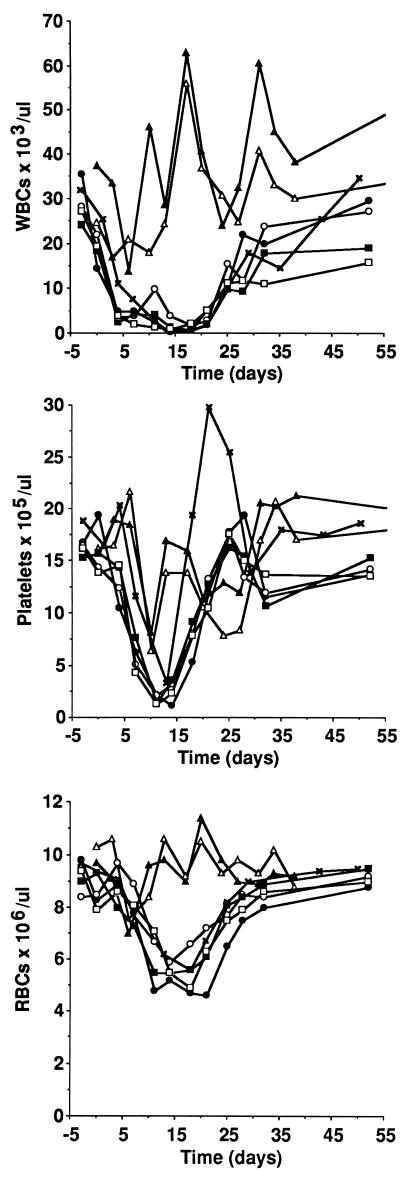
Blood counts remained stable in the untreated control group. There was a moderate but brief decrease in white blood cell and platelet counts among control groups that received only Taxol (Fig. 3). Myelotoxicity was lesser in extent and duration in the group receiving 90Y-ChL6 alone than among groups that received 90Y-ChL6 and Taxol. In all groups, the platelet count began to recover after 14 days; the white blood cell count began to recover after 21 days; red blood cell counts declined less and began to recover after 21 days.
Figure 3.
White blood cell, platelet, and red blood cell counts in mice. Myelotoxicity was lesser in extent and duration in the group receiving 90Y-ChL6 alone than among groups that received 90Y-ChL6 and Taxol. Addition of Taxol had a moderate but brief effect on white blood cell or red blood cell counts. 90Y-ChL6, ×; 300 μg of Taxol, ▴; 600 μg of Taxol, ▵; 300 μg of Taxol given 24 hours before 90Y-ChL6, •; 600 μg of Taxol given 24 hours before 90Y-ChL6, ○; 600 μg of Taxol given 6 hours after 90Y-ChL6, ▪; 600 μg of Taxol given 24 hours after 90Y-ChL6, □.
Mortality.
Six mice died within 30 days of therapy; five of the deaths were in control groups that received no 90Y-ChL6 (Table 1). The deaths were randomly distributed [Fisher’s Exact test, P = 0.2 (29)] among the groups.
DISCUSSION
The intent of this study was to examine the potential of Taxol as a synergistic agent with 90Y-ChL6 for therapy of breast cancer. Using the concept of a response surface model, a series of experiments were performed to evaluate the optimal dose and timing of Taxol relative to 90Y-ChL6. The results of each experiment in this series were evaluated to determine parameters for the next experiment in an effort to find the combination of variables likely to produce the best therapeutic effect. The experiments were not designed to prove one regimen statistically better than all others but to assure that the regimen selected was not substantially worse than the optimal and to determine whether any regimen showed sufficient promise for future work (30, 31). ChL6 was chosen for the radioimmunoconjugate because ChL6 stained 75% of cancer cells in 48% of patients with metastatic breast cancers when tested by immunopathology (32). ChL6 also reacts with the majority of cells in HBT 3477 tumors grown subcutaneously in nude mice (11).
Enhancement of therapeutic effect when Taxol was added to 90Y-ChL6 therapy for HBT 3477 xenografts was striking (Table 1). When Taxol was administered 6 or 24 hours after 90Y-ChL6, 100% (46 of 46) of tumors responded and 48% (22 of 46) were cured. These results were better than when Taxol was given prior to 90Y-ChL6 (P = 0.001; Fig. 2). There was no statistically significant therapeutic enhancement when 600 μg of Taxol was given versus 300 μg. No therapeutic response to Taxol alone was demonstrated in the HBT 3477 mouse model. When Taxol was given 24 hours after ChL6 without 90Y, only 25% (3 of 12) of tumors achieved partial regression and there were no complete regressions or cures, thereby demonstrating that tumor-targeted radiation was essential for optimal therapeutic efficacy of 90Y-ChL6/Taxol. Information from previous studies of tumor uptake indicate that 9.6 MBq of 90Y-ChL6 delivers a radiation dose of 26.3–41.4 Gy to tumors (12).
The synergistic therapeutic effect of Taxol with 90Y-ChL6 may relate in part to the p53 mutant status and BCL2 expression in HBT 3477 cells, observations that increase the likelihood that the results of our study are relevant to therapy for breast cancer in patients. Mutant p53 and BCL2 expression have been increasingly associated with breast cancer in patients (1, 4, 5). p53 monitors DNA damage and interrupts cells in G1 phase, providing time for their repair. Cells lacking functional p53 fail to pause in G1 phase after DNA damage, are resistant to death from DNA damaging agents, and are frequently unable to initiate apoptotic cell death (1–3). Both p53-dependent and p53-independent apoptosis are inhibited by BCL2 (2, 13). Taxol induces G2/M arrest and blocks BCL2 by phosphorylation followed by p53-independent apoptosis, and cells lacking functional p53 are more sensitive to Taxol-induced G2/M block (2, 14, 15, 17). We propose that the mechanisms enabling the 90Y-ChL6/Taxol combination to be synergistic involve (i) death of p53 mutant cancer cells through Taxol blockage of BCL2 and induction of p53-independent apoptosis, which is enhanced by 90Y; and (ii) death of wild-type p53 cancer cells due to radiation-induced DNA damage further enhanced by Taxol (33–35). Normal cells receive much lower doses of radiation than the cancer cells; at these low doses of radiation and Taxol, sublethal DNA damage can be repaired.
The goal of cancer therapy is to spare normal cells while damaging cancer cells sufficiently so that cells not killed outright undergo apoptosis. This goal has not been achieved because standard cytotoxic agents cause excessive toxicity to normal tissues. Our results suggest that the previously demonstrated therapeutic efficacy of 90Y-ChL6 alone in a mouse model of breast cancer can be significantly enhanced by low doses of Taxol given after 90Y has substantially cleared from normal tissues, thereby limiting toxicity.
In conclusion, 90Y-ChL6 and Taxol can be given in a sequence that enhances therapeutic efficacy. Over time after injection, 90Y-ChL6 binds to malignant cells as it circulates and unbound 90Y-ChL6 is cleared from normal tissues. Thus, a “window” in time exists when there is ongoing tumor irradiation but little concurrent normal tissue irradiation. Given in this window, Taxol, a small molecule rapidly taken up by the cancer, enhanced the therapeutic effect of 90Y-ChL6 on targeted malignant cells. The optimal time for Taxol administration in this xenograft model was 6–24 hours after 90Y-ChL6. The HBT 3477 cells used in this study are relatively radioresistant, possess a p53 mutation resulting in a nonfunctional protein, express BCL2, and are highly anaplastic. Such properties are frequently present in human breast cancer that is currently incurable by standard therapies. The therapeutic response of aggressive HBT 3477 tumors to Taxol and 90Y-ChL6 warrants studies of these agents in other models and in patients.
Acknowledgments
We thank Dr. Edith Perez for her scientific suggestions and Dr. David Zava at Aeron Corporation for information on HBT 3477 cell reactivity. This research was supported by grants from the National Cancer Institute (CA47829 and PHS CA16861) and the Department of Energy (DE-FG03-84ER60233).
ABBREVIATIONS
- DOTA
1,4,7,10-tetraazacyclododecane-N,N′,N",N‴-tetraacetic acid
- ChL6
chimeric L6 antibody
- 90Y-ChL6
90Y-labeled DOTA-peptide-ChL6
References
- 1.Levine A J, Momand J, Finlay C A. Nature (London) 1991;351:453–456. doi: 10.1038/351453a0. [DOI] [PubMed] [Google Scholar]
- 2.Harris C C. J Natl Cancer Inst. 1996;88:1442–1455. doi: 10.1093/jnci/88.20.1442. [DOI] [PubMed] [Google Scholar]
- 3.Hollstein M, Sidransky D, Vogelstein B, Harris C C. Science. 1991;253:49–53. doi: 10.1126/science.1905840. [DOI] [PubMed] [Google Scholar]
- 4.Lane D P. Nature (London) 1992;358:15–16. doi: 10.1038/358015a0. [DOI] [PubMed] [Google Scholar]
- 5.Leek R D, Kaklamanis L, Pezzella F, Gatter K C, Harris A L. Br J Cancer. 1994;69:135–139. doi: 10.1038/bjc.1994.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parker S L, Tong T, Bolden S, Wingo P A. CA-Cancer J Clin. 1996;65:5–27. doi: 10.3322/canjclin.46.1.5. [DOI] [PubMed] [Google Scholar]
- 7.Wilder R B, DeNardo G L, DeNardo S J. J Clin Oncol. 1996;14:1383–1400. doi: 10.1200/JCO.1996.14.4.1383. [DOI] [PubMed] [Google Scholar]
- 8.DeNardo S J, O’Grady L F, Macey D J, Kroger L A, DeNardo G L, Lamborn K R, Levy N B, Mills S L, Hellström I, Hellström K E. Nucl Med Biol. 1991;18:621–631. doi: 10.1016/0883-2897(91)90032-g. [DOI] [PubMed] [Google Scholar]
- 9.DeNardo S J, Mirick G R, Kroger L A, O’Grady L F, Erickson K L, Yuan A, Lamborn K R, Hellström I, Hellström K E, DeNardo G L. Cancer. 1994;73:1023–1032. doi: 10.1002/1097-0142(19940201)73:3+<1023::aid-cncr2820731341>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 10.DeNardo S J, O’Grady L F, Richman C M, DeNardo G L. In: Antigen and Antibody Molecular Engineering in Breast Cancer Diagnosis and Treatment. Ceriani R L, editor. New York: Plenum; 1994. pp. 203–211. [Google Scholar]
- 11.DeNardo S J, Zhong G-R, Salako Q, Li M, DeNardo G L, Meares C F. J Nucl Med. 1995;36:829–836. [PubMed] [Google Scholar]
- 12.DeNardo S J, Gumerlock P H, Winthrop M D, Mack P C, Chi S G, Lamborn K R, Shen S, Miers L A, deVere White R W, DeNardo G L. Cancer Res. 1995;55:5837–5841. [PubMed] [Google Scholar]
- 13.Strasser A, Harris A W, Jacks T, Cory S. Cell. 1994;79:329–339. doi: 10.1016/0092-8674(94)90201-1. [DOI] [PubMed] [Google Scholar]
- 14.Wahl A F, Donaldson K L, Fairchild C, Lee F Y F, Foster S A, Demers G W, Galloway D A. Nat Med. 1996;2:72–79. doi: 10.1038/nm0196-72. [DOI] [PubMed] [Google Scholar]
- 15.Vogelstein G. Nature (London) 1990;348:681–682. doi: 10.1038/348681a0. [DOI] [PubMed] [Google Scholar]
- 16.Horwitz S B. Trends Pharmacol Sci. 1992;13:134–136. doi: 10.1016/0165-6147(92)90048-b. [DOI] [PubMed] [Google Scholar]
- 17.Hadler S, Chintapalli J, Croce C M. Cancer Res. 1996;56:1253–1255. [PubMed] [Google Scholar]
- 18.Liu A Y, Robinson R R, Hellström K E, Murray E D, Chang C P, Hellström I. Proc Natl Acad Sci USA. 1987;84:3439–3443. doi: 10.1073/pnas.84.10.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hellström I, Horn D, Linsley P, Brown J P, Brankovan V, Hellström K E. Cancer Res. 1986;46:3917–3923. [PubMed] [Google Scholar]
- 20.Marken J S, Bajorath J, Edwards C P, Farr A G, Schieven G L, Hellström I, Hellström K E, Aruffo A. J Biol Chem. 1994;269:7397–7401. [PubMed] [Google Scholar]
- 21.Freireich E J, Gehan E A, Rall D P, Schmidt L H, Skipper H E. Cancer Chemother Rep. 1966;50:219–244. [PubMed] [Google Scholar]
- 22.Hortobagyi G N, Holmes F A. Semin Oncol. 1996;23:4–9. [PubMed] [Google Scholar]
- 23.Mattes M J, Major P P, Goldenberg D M, Dion A S, Hunter R V P, Klein K M. Cancer Res. 1990;50:880–884. [PubMed] [Google Scholar]
- 24.Winthrop, M. D., DeNardo, S. J., Muenzer, J. T., Chi, S. G. & Gumerlock, P. H. (1997) Cancer, in press. [DOI] [PubMed]
- 25.Li A, Meares C F, Salako Q, Kukis D L, Zhong G-R, Miers L A, DeNardo S J. Cancer Res. 1995;55:5726s–5728s. [PubMed] [Google Scholar]
- 26.DeNardo G L, Kukis D L, Shen S, Mausner L F, Meares C F, Srivastava S C, Miers L A, DeNardo S J. Clin Cancer Res. 1996;3:71–79. [PubMed] [Google Scholar]
- 27.Seiverd C. Hematology for Medical Technologists. 4th Ed. Philadelphia: Lea Febiger; 1972. pp. 91–432. [Google Scholar]
- 28.Agresti A. Categorical Data Analysis. New York: Wiley; 1990. pp. 283–287. [Google Scholar]
- 29.Agresti A. Categorical Data Analysis. New York: Wiley; 1990. p. 60. [Google Scholar]
- 30.Carter W H, Jr, Wampler G L. Cancer Treat Rep. 1986;70:133–140. [PubMed] [Google Scholar]
- 31.Berenbaum M C. Br J Cancer. 1990;61:101–109. doi: 10.1038/bjc.1990.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Howell L P, DeNardo S J, Levy N B, Lund J, DeNardo G L. Int J Biol Markers. 1995;10:129–135. doi: 10.1177/172460089501000301. [DOI] [PubMed] [Google Scholar]
- 33.Liebmann J, Cook J A, Fisher J, Teague D, Mitchell J B. Int J Radiat Oncol Biol Phys. 1994;29:559–564. doi: 10.1016/0360-3016(94)90456-1. [DOI] [PubMed] [Google Scholar]
- 34.Minarik L, Hall E J. Radiother Oncol. 1994;32:124–128. doi: 10.1016/0167-8140(94)90098-1. [DOI] [PubMed] [Google Scholar]
- 35.Balakrishna L L, Ferrell S M, Block N L. Anticancer Res. 1995;15:93–98. [PubMed] [Google Scholar]