Abstract
Trehalose-6-P inhibits hexokinases in Saccharomyces cerevisiae (M. A. Blázquez, R. Lagunas, C. Gancedo, and J. M. Gancedo, FEBS Lett. 329:51-54, 1993), and disruption of the TPS1 gene (formerly named CIF1 or FDP1) encoding trehalose-6-P synthase prevents growth in glucose. We have found that the hexokinase from Schizosaccharomyces pombe is not inhibited by trehalose-6-P even at a concentration of 3 mM. The highest internal concentration of trehalose-6-P that we measured in S. pombe was 0.75 mM after heat shock. We have isolated from S. pombe the tps1+ gene, which is homologous to the Saccharomyces cerevisiae TPS1 gene. The DNA sequence from tps1+ predicts a protein of 479 amino acids with 65% identity with the protein of S. cerevisiae. The tps1+ gene expressed from its own promoter could complement the lack of trehalose-6-P synthase in S. cerevisiae tps1 mutants. The TPS1 gene from S. cerevisiae could also restore trehalose synthesis in S. pombe tps1 mutants. A chromosomal disruption of the tps1+ gene in S. pombe did not have a noticeable effect on growth in glucose, in contrast with the disruption of TPS1 in S. cerevisiae. However, the disruption prevented germination of spores carrying it. The level of an RNA hybridizing with an internal probe of the tps1+ gene reached a maximum after 20 min of heat shock treatment. The results presented support the idea that trehalose-6-P plays a role in the control of glycolysis in S. cerevisiae but not in S. pombe and show that the trehalose pathway has different roles in the two yeast species.
Full text
PDF

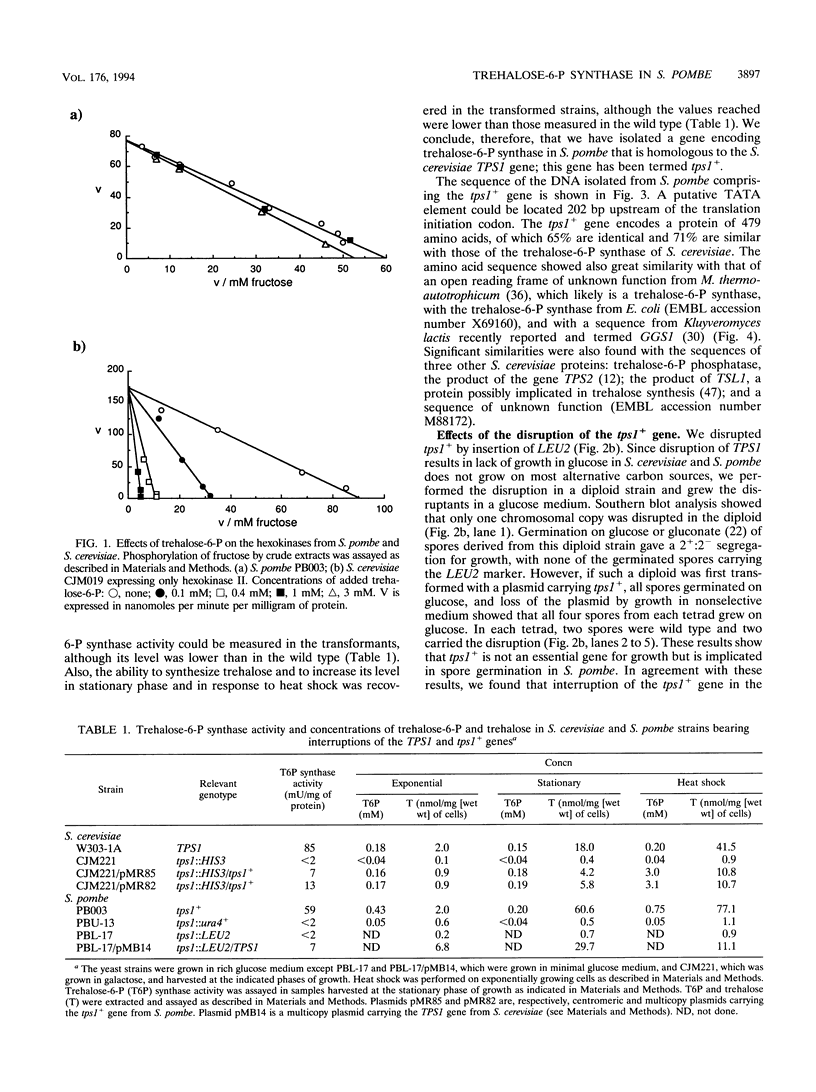
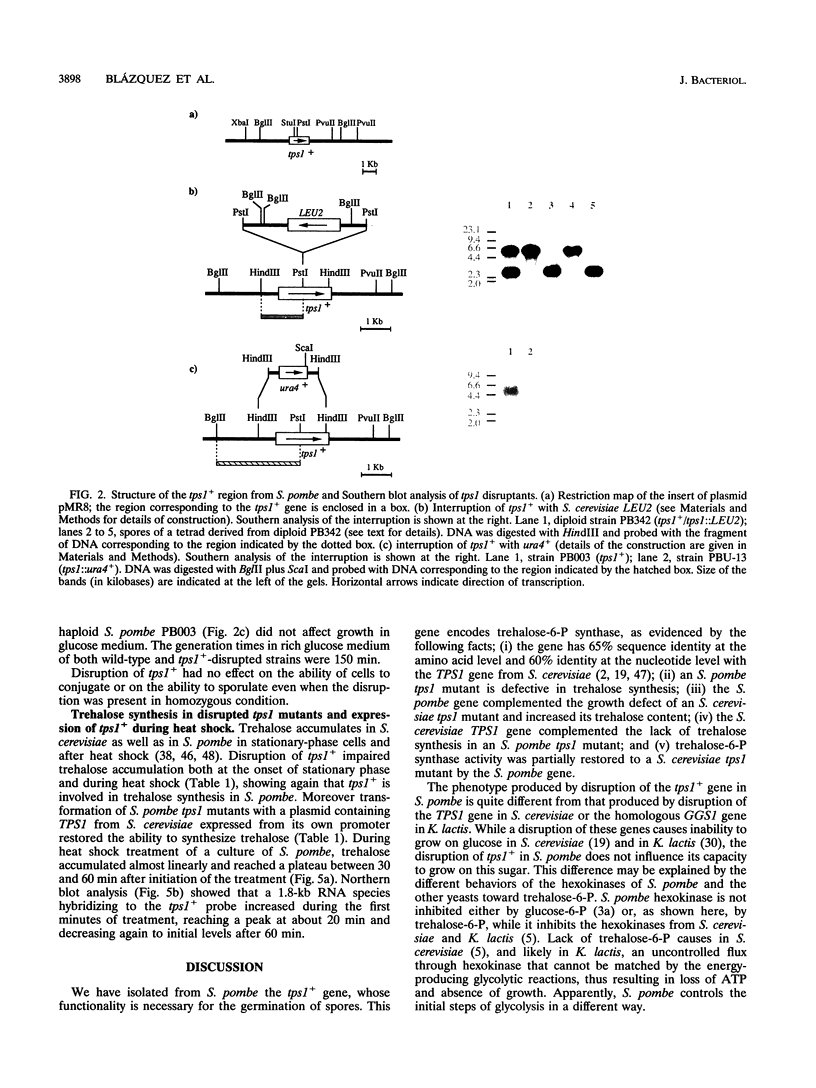


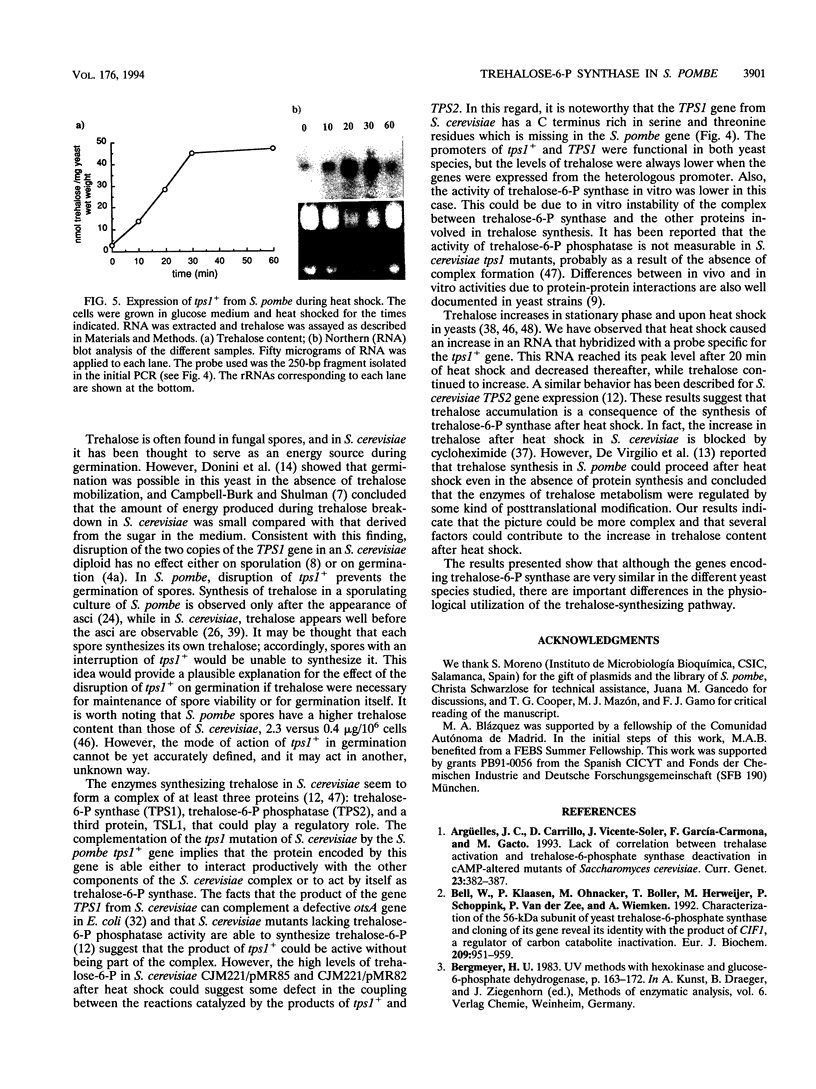

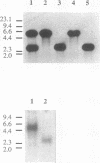
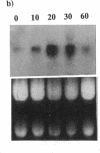
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Argüelles J. C., Carrillo D., Vicente-Soler J., García-Carmona F., Gacto M. Lack of correlation between trehalase activation and trehalose-6 phosphate synthase deactivation in cAMP-altered mutants of Saccharomyces cerevisiae. Curr Genet. 1993 May-Jun;23(5-6):382–387. doi: 10.1007/BF00312622. [DOI] [PubMed] [Google Scholar]
- Bell W., Klaassen P., Ohnacker M., Boller T., Herweijer M., Schoppink P., Van der Zee P., Wiemken A. Characterization of the 56-kDa subunit of yeast trehalose-6-phosphate synthase and cloning of its gene reveal its identity with the product of CIF1, a regulator of carbon catabolite inactivation. Eur J Biochem. 1992 Nov 1;209(3):951–959. doi: 10.1111/j.1432-1033.1992.tb17368.x. [DOI] [PubMed] [Google Scholar]
- Blázquez M. A., Gancedo C. Identification of extragenic suppressors of the cif1 mutation in Saccharomyces cerevisiae. Curr Genet. 1994 Feb;25(2):89–94. doi: 10.1007/BF00309531. [DOI] [PubMed] [Google Scholar]
- Blázquez M. A., Lagunas R., Gancedo C., Gancedo J. M. Trehalose-6-phosphate, a new regulator of yeast glycolysis that inhibits hexokinases. FEBS Lett. 1993 Aug 23;329(1-2):51–54. doi: 10.1016/0014-5793(93)80191-v. [DOI] [PubMed] [Google Scholar]
- Broach J. R., Strathern J. N., Hicks J. B. Transformation in yeast: development of a hybrid cloning vector and isolation of the CAN1 gene. Gene. 1979 Dec;8(1):121–133. doi: 10.1016/0378-1119(79)90012-x. [DOI] [PubMed] [Google Scholar]
- Campbell-Burk S. L., Shulman R. G. High-resolution NMR studies of Saccharomyces cerevisiae. Annu Rev Microbiol. 1987;41:595–616. doi: 10.1146/annurev.mi.41.100187.003115. [DOI] [PubMed] [Google Scholar]
- Cannon J. F., Pringle J. R., Fiechter A., Khalil M. Characterization of glycogen-deficient glc mutants of Saccharomyces cerevisiae. Genetics. 1994 Feb;136(2):485–503. doi: 10.1093/genetics/136.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifton D., Fraenkel D. G. Mutant studies of yeast phosphofructokinase. Biochemistry. 1982 Apr 13;21(8):1935–1942. doi: 10.1021/bi00537a037. [DOI] [PubMed] [Google Scholar]
- De Virgilio C., Bürckert N., Bell W., Jenö P., Boller T., Wiemken A. Disruption of TPS2, the gene encoding the 100-kDa subunit of the trehalose-6-phosphate synthase/phosphatase complex in Saccharomyces cerevisiae, causes accumulation of trehalose-6-phosphate and loss of trehalose-6-phosphate phosphatase activity. Eur J Biochem. 1993 Mar 1;212(2):315–323. doi: 10.1111/j.1432-1033.1993.tb17664.x. [DOI] [PubMed] [Google Scholar]
- De Virgilio C., Simmen U., Hottiger T., Boller T., Wiemken A. Heat shock induces enzymes of trehalose metabolism, trehalose accumulation, and thermotolerance in Schizosaccharomyces pombe, even in the presence of cycloheximide. FEBS Lett. 1990 Oct 29;273(1-2):107–110. doi: 10.1016/0014-5793(90)81062-s. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnini C., Puglisi P. P., Vecli A., Marmiroli N. Germination of Saccharomyces cerevisiae ascospores without trehalose mobilization as revealed by in vivo 13C nuclear magnetic resonance spectroscopy. J Bacteriol. 1988 Aug;170(8):3789–3791. doi: 10.1128/jb.170.8.3789-3791.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder R. T., Loh E. Y., Davis R. W. RNA from the yeast transposable element Ty1 has both ends in the direct repeats, a structure similar to retrovirus RNA. Proc Natl Acad Sci U S A. 1983 May;80(9):2432–2436. doi: 10.1073/pnas.80.9.2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Gancedo J. M., Clifton D., Fraenkel D. G. Yeast hexokinase mutants. J Biol Chem. 1977 Jul 10;252(13):4443–4444. [PubMed] [Google Scholar]
- González M. I., Stucka R., Blázquez M. A., Feldmann H., Gancedo C. Molecular cloning of CIF1, a yeast gene necessary for growth on glucose. Yeast. 1992 Mar;8(3):183–192. doi: 10.1002/yea.320080304. [DOI] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill J. E., Myers A. M., Koerner T. J., Tzagoloff A. Yeast/E. coli shuttle vectors with multiple unique restriction sites. Yeast. 1986 Sep;2(3):163–167. doi: 10.1002/yea.320020304. [DOI] [PubMed] [Google Scholar]
- Hoffman C. S., Winston F. A ten-minute DNA preparation from yeast efficiently releases autonomous plasmids for transformation of Escherichia coli. Gene. 1987;57(2-3):267–272. doi: 10.1016/0378-1119(87)90131-4. [DOI] [PubMed] [Google Scholar]
- Inoue H., Shimoda C. Induction of trehalase activity on a nitrogen-free medium: a sporulation-specific event in the fission yeast, Schizosaccharomyces pombe. Mol Gen Genet. 1981;183(1):32–36. doi: 10.1007/BF00270134. [DOI] [PubMed] [Google Scholar]
- Ito H., Fukuda Y., Murata K., Kimura A. Transformation of intact yeast cells treated with alkali cations. J Bacteriol. 1983 Jan;153(1):163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane S. M., Roth R. Carbohydrate metabolism during ascospore development in yeast. J Bacteriol. 1974 Apr;118(1):8–14. doi: 10.1128/jb.118.1.8-14.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kienle I., Burgert M., Holzer H. Assay of trehalose with acid trehalase purified from Saccharomyces cerevisiae. Yeast. 1993 Jun;9(6):607–611. doi: 10.1002/yea.320090607. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Londesborough J., Vuorio O. E. Purification of trehalose synthase from baker's yeast. Its temperature-dependent activation by fructose 6-phosphate and inhibition by phosphate. Eur J Biochem. 1993 Sep 15;216(3):841–848. doi: 10.1111/j.1432-1033.1993.tb18206.x. [DOI] [PubMed] [Google Scholar]
- Luyten K., de Koning W., Tesseur I., Ruiz M. C., Ramos J., Cobbaert P., Thevelein J. M., Hohmann S. Disruption of the Kluyveromyces lactis GGS1 gene causes inability to grow on glucose and fructose and is suppressed by mutations that reduce sugar uptake. Eur J Biochem. 1993 Oct 15;217(2):701–713. doi: 10.1111/j.1432-1033.1993.tb18296.x. [DOI] [PubMed] [Google Scholar]
- Maundrell K. Thiamine-repressible expression vectors pREP and pRIP for fission yeast. Gene. 1993 Jan 15;123(1):127–130. doi: 10.1016/0378-1119(93)90551-d. [DOI] [PubMed] [Google Scholar]
- McDougall J., Kaasen I., Strøm A. R. A yeast gene for trehalose-6-phosphate synthase and its complementation of an Escherichia coli otsA mutant. FEMS Microbiol Lett. 1993 Feb 15;107(1):25–30. doi: 10.1016/0378-1097(93)90348-6. [DOI] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Moreno S., Klar A., Nurse P. Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol. 1991;194:795–823. doi: 10.1016/0076-6879(91)94059-l. [DOI] [PubMed] [Google Scholar]
- Navon G., Shulman R. G., Yamane T., Eccleshall T. R., Lam K. B., Baronofsky J. J., Marmur J. Phosphorus-31 nuclear magnetic resonance studies of wild-type and glycolytic pathway mutants of Saccharomyces cerevisiae. Biochemistry. 1979 Oct 16;18(21):4487–4499. doi: 10.1021/bi00588a006. [DOI] [PubMed] [Google Scholar]
- Panek A. C., Vânia J. J., Paschoalin M. F., Panek D. Regulation of trehalose metabolism in Saccharomyces cerevisiae mutants during temperature shifts. Biochimie. 1990 Jan;72(1):77–79. doi: 10.1016/0300-9084(90)90176-h. [DOI] [PubMed] [Google Scholar]
- Roth R. Carbohydrate accumulation during the sporulation of yeast. J Bacteriol. 1970 Jan;101(1):53–57. doi: 10.1128/jb.101.1.53-57.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell P. R., Hall B. D. Structure of the Schizosaccharomyces pombe cytochrome c gene. Mol Cell Biol. 1982 Feb;2(2):106–116. doi: 10.1128/mcb.2.2.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikorski R. S., Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989 May;122(1):19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas B. J., Rothstein R. Elevated recombination rates in transcriptionally active DNA. Cell. 1989 Feb 24;56(4):619–630. doi: 10.1016/0092-8674(89)90584-9. [DOI] [PubMed] [Google Scholar]
- Vuorio O. E., Kalkkinen N., Londesborough J. Cloning of two related genes encoding the 56-kDa and 123-kDa subunits of trehalose synthase from the yeast Saccharomyces cerevisiae. Eur J Biochem. 1993 Sep 15;216(3):849–861. doi: 10.1111/j.1432-1033.1993.tb18207.x. [DOI] [PubMed] [Google Scholar]
- Wiemken A. Trehalose in yeast, stress protectant rather than reserve carbohydrate. Antonie Van Leeuwenhoek. 1990 Oct;58(3):209–217. doi: 10.1007/BF00548935. [DOI] [PubMed] [Google Scholar]
- Woods D. R., Reid S. J. Recent developments on the regulation and structure of glutamine synthetase enzymes from selected bacterial groups. FEMS Microbiol Rev. 1993 Aug;11(4):273–283. doi: 10.1111/j.1574-6976.1993.tb00001.x. [DOI] [PubMed] [Google Scholar]
- van de Poll K. W., Kerkenaar A., Schamhart D. H. Isolation of a regulatory mutant of fructose-1,6-diphosphatase in Saccharomyces carlsbergensis. J Bacteriol. 1974 Mar;117(3):965–970. doi: 10.1128/jb.117.3.965-970.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]