Abstract
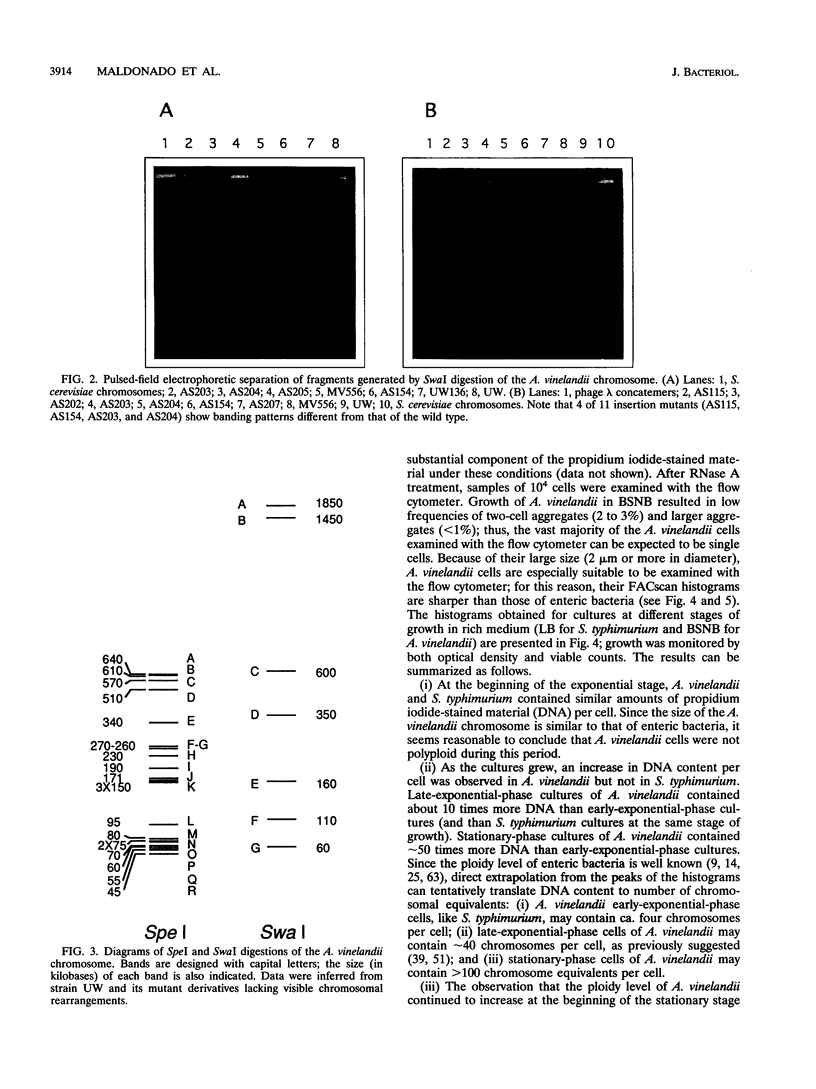
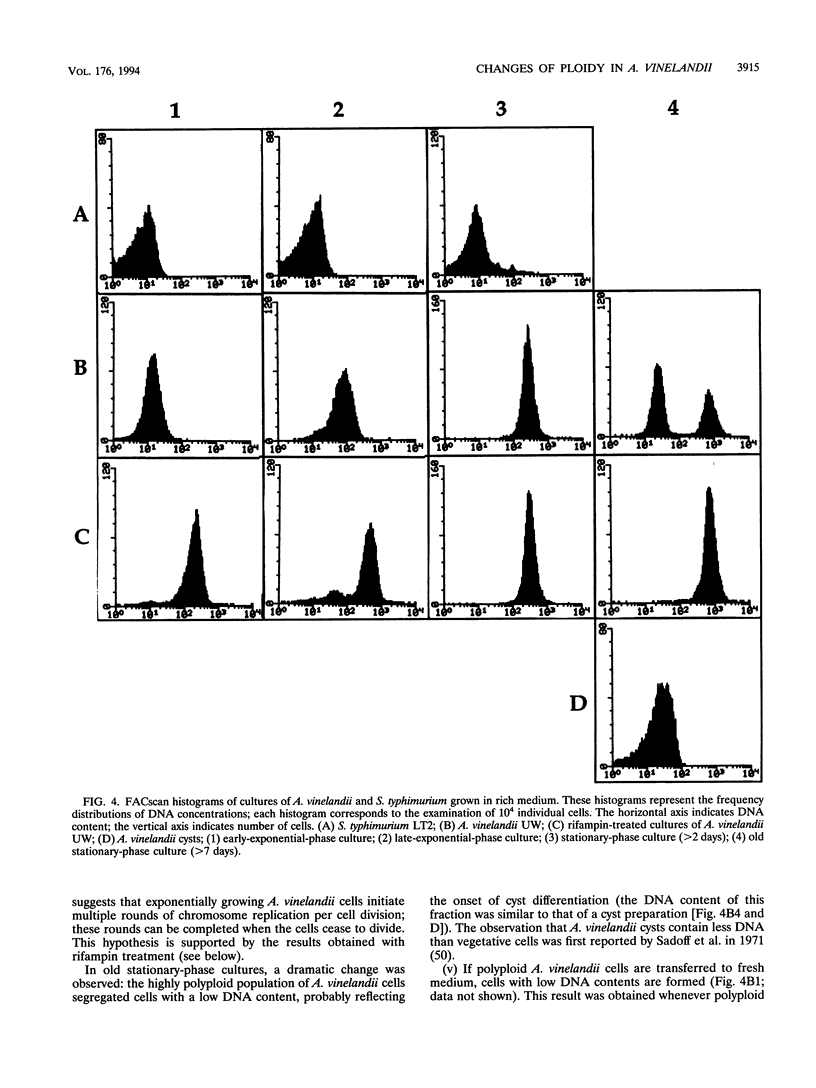
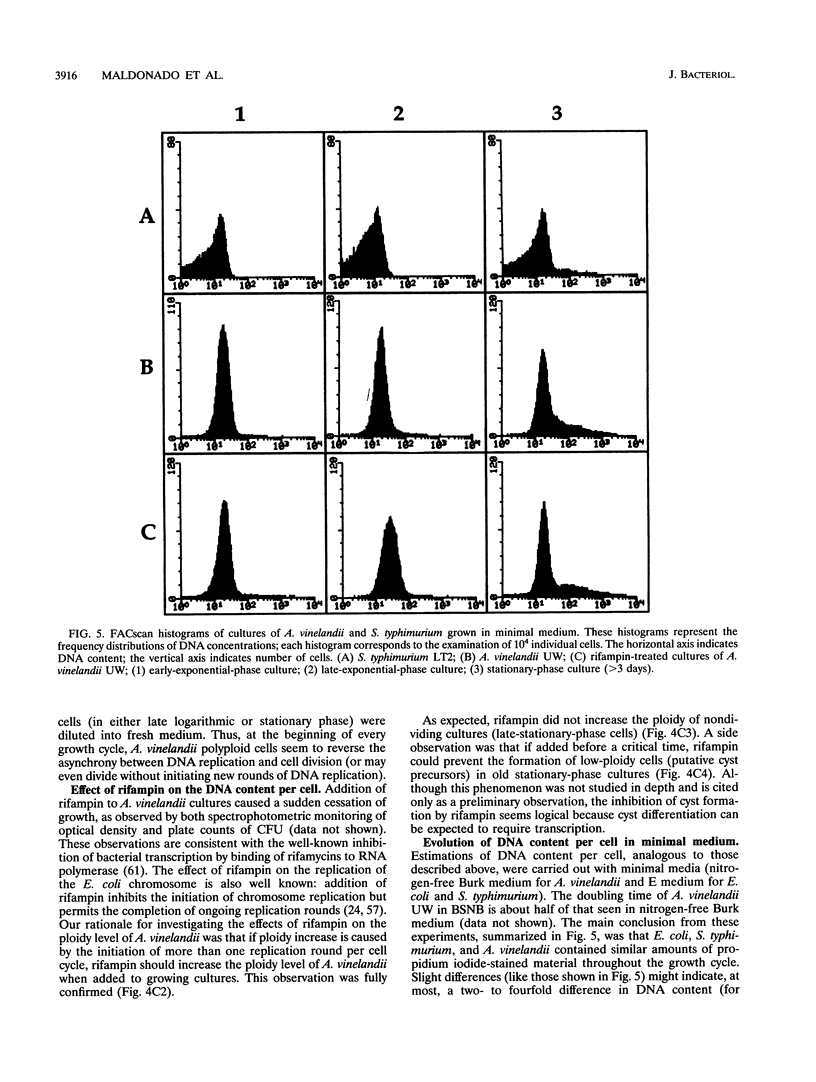
The size of the Azotobacter vinelandii chromosome is approximately 4,700 kb, as calculated by pulsed-field electrophoretic separation of fragments digested with the rarely cutting endonucleases SpeI and SwaI. Surveys of DNA content per cell by flow cytometry indicated the existence of ploidy changes during the A. vinelandii growth cycle in rich medium. Early-exponential-phase cells have a ploidy level similar to that of Escherichia coli or Salmonella typhimurium (probably ca. four chromosomes per cell), but a continuous increase of DNA content per cell is observed during growth. Late-exponential-phase cells may contain > 40 chromosomes per cell, while cells in the early stationary stage may contain > 80 chromosomes per cell. In late-stationary-phase cultures, the DNA content per cell is even higher, probably over 100 chromosome equivalents per cell. A dramatic change is observed in old stationary-phase cultures, when the population of highly polyploid bacteria segregates cells with low ploidy. The DNA content of the latter cells resembles that of cysts, suggesting that the process may reflect the onset of cyst differentiation. Cells with low ploidy are also formed when old stationary-phase cultures are diluted into fresh medium. Addition of rifampin to exponential-phase cultures causes a rapid increase in DNA content, indicating that A. vinelandii initiates multiple rounds of chromosome replication per cell division. Growth in minimal medium does not result in the spectacular changes of ploidy observed during rapid growth; this observation suggests that the polyploidy of A. vinelandii may not exist outside the laboratory.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett L. T., Cannon F., Dean D. R. Nucleotide sequence and mutagenesis of the nifA gene from Azotobacter vinelandii. Mol Microbiol. 1988 May;2(3):315–321. doi: 10.1111/j.1365-2958.1988.tb00034.x. [DOI] [PubMed] [Google Scholar]
- Birren B. W., Lai E., Clark S. M., Hood L., Simon M. I. Optimized conditions for pulsed field gel electrophoretic separations of DNA. Nucleic Acids Res. 1988 Aug 11;16(15):7563–7582. doi: 10.1093/nar/16.15.7563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco G., Drummond M., Woodley P., Kennedy C. Sequence and molecular analysis of the nifL gene of Azotobacter vinelandii. Mol Microbiol. 1993 Aug;9(4):869–879. doi: 10.1111/j.1365-2958.1993.tb01745.x. [DOI] [PubMed] [Google Scholar]
- Blanco G., Ramos F., Medina J. R., Tortolero M. A chromosomal linkage map of Azotobacter vinelandii. Mol Gen Genet. 1990 Nov;224(2):241–247. doi: 10.1007/BF00271557. [DOI] [PubMed] [Google Scholar]
- Chu G., Vollrath D., Davis R. W. Separation of large DNA molecules by contour-clamped homogeneous electric fields. Science. 1986 Dec 19;234(4783):1582–1585. doi: 10.1126/science.3538420. [DOI] [PubMed] [Google Scholar]
- Contreras A., Drummond M., Bali A., Blanco G., Garcia E., Bush G., Kennedy C., Merrick M. The product of the nitrogen fixation regulatory gene nfrX of Azotobacter vinelandii is functionally and structurally homologous to the uridylyltransferase encoded by glnD in enteric bacteria. J Bacteriol. 1991 Dec;173(24):7741–7749. doi: 10.1128/jb.173.24.7741-7749.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras A., Maldonado R., Casadesus J. Tn5 mutagenesis and insertion replacement in Azotobacter vinelandii. Plasmid. 1991 Jan;25(1):76–80. doi: 10.1016/0147-619x(91)90009-l. [DOI] [PubMed] [Google Scholar]
- Contreras A, Casadesús J. Tn10 mutagenesis in Azotobacter vinelandii. Mol Gen Genet. 1987 Sep;209(2):276–282. doi: 10.1007/BF00329654. [DOI] [PubMed] [Google Scholar]
- Guerrero M. G., Vega J. M., Leadbetter E., Losada M. Preparation and characterization of a soluble nitrate reductase from Azotobacter chroococcum. Arch Mikrobiol. 1973 Jun 25;91(4):287–304. doi: 10.1007/BF00425049. [DOI] [PubMed] [Google Scholar]
- Hanemaaijer R., Janssen A., de Kok A., Veeger C. The dihydrolipoyltransacetylase component of the pyruvate dehydrogenase complex from Azotobacter vinelandii. Molecular cloning and sequence analysis. Eur J Biochem. 1988 Jul 1;174(4):593–599. doi: 10.1111/j.1432-1033.1988.tb14140.x. [DOI] [PubMed] [Google Scholar]
- Heath J. D., Perkins J. D., Sharma B., Weinstock G. M. NotI genomic cleavage map of Escherichia coli K-12 strain MG1655. J Bacteriol. 1992 Jan;174(2):558–567. doi: 10.1128/jb.174.2.558-567.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmstetter C. E. DNA synthesis during the division cycle of rapidly growing Escherichia coli B/r. J Mol Biol. 1968 Feb 14;31(3):507–518. doi: 10.1016/0022-2836(68)90424-5. [DOI] [PubMed] [Google Scholar]
- Hitchins V. M., Sadoff H. L. Sequential metabolic events during encystment of Azobacter vinelandii. J Bacteriol. 1973 Mar;113(3):1273–1279. doi: 10.1128/jb.113.3.1273-1279.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itaya M., Tanaka T. Complete physical map of the Bacillus subtilis 168 chromosome constructed by a gene-directed mutagenesis method. J Mol Biol. 1991 Aug 5;220(3):631–648. doi: 10.1016/0022-2836(91)90106-g. [DOI] [PubMed] [Google Scholar]
- Jacobson M. R., Brigle K. E., Bennett L. T., Setterquist R. A., Wilson M. S., Cash V. L., Beynon J., Newton W. E., Dean D. R. Physical and genetic map of the major nif gene cluster from Azotobacter vinelandii. J Bacteriol. 1989 Feb;171(2):1017–1027. doi: 10.1128/jb.171.2.1017-1027.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joerger R. D., Bishop P. E. Nucleotide sequence and genetic analysis of the nifB-nifQ region from Azotobacter vinelandii. J Bacteriol. 1988 Apr;170(4):1475–1487. doi: 10.1128/jb.170.4.1475-1487.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joerger R. D., Jacobson M. R., Bishop P. E. Two nifA-like genes required for expression of alternative nitrogenases by Azotobacter vinelandii. J Bacteriol. 1989 Jun;171(6):3258–3267. doi: 10.1128/jb.171.6.3258-3267.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joerger R. D., Jacobson M. R., Premakumar R., Wolfinger E. D., Bishop P. E. Nucleotide sequence and mutational analysis of the structural genes (anfHDGK) for the second alternative nitrogenase from Azotobacter vinelandii. J Bacteriol. 1989 Feb;171(2):1075–1086. doi: 10.1128/jb.171.2.1075-1086.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joerger R. D., Loveless T. M., Pau R. N., Mitchenall L. A., Simon B. H., Bishop P. E. Nucleotide sequences and mutational analysis of the structural genes for nitrogenase 2 of Azotobacter vinelandii. J Bacteriol. 1990 Jun;172(6):3400–3408. doi: 10.1128/jb.172.6.3400-3408.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy C., Toukdarian A. Genetics of azotobacters: applications to nitrogen fixation and related aspects of metabolism. Annu Rev Microbiol. 1987;41:227–258. doi: 10.1146/annurev.mi.41.100187.001303. [DOI] [PubMed] [Google Scholar]
- Krawiec S., Riley M. Organization of the bacterial chromosome. Microbiol Rev. 1990 Dec;54(4):502–539. doi: 10.1128/mr.54.4.502-539.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LARK K. G., MAALØE O. The induction of cellular and nuclear division in Salmonella typhimurium by means of temperature shifts. Biochim Biophys Acta. 1954 Nov;15(3):345–356. doi: 10.1016/0006-3002(54)90036-0. [DOI] [PubMed] [Google Scholar]
- Lark K. G. Evidence for the direct involvement of RNA in the initiation of DNA replication in Escherichia coli 15T. J Mol Biol. 1972 Feb 28;64(1):47–60. doi: 10.1016/0022-2836(72)90320-8. [DOI] [PubMed] [Google Scholar]
- Le O., Shen B., Iismaa S. E., Burgess B. K. Azotobacter vinelandii mutS: nucleotide sequence and mutant analysis. J Bacteriol. 1993 Dec;175(23):7707–7710. doi: 10.1128/jb.175.23.7707-7710.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L. P., Sadoff H. L. Encystment and polymer production by Azotobacter vinelandii in the presence of beta-hydroxybutyrate. J Bacteriol. 1968 Jun;95(6):2336–2343. doi: 10.1128/jb.95.6.2336-2343.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S. L., Hessel A., Sanderson K. E. The XbaI-BlnI-CeuI genomic cleavage map of Salmonella typhimurium LT2 determined by double digestion, end labelling, and pulsed-field gel electrophoresis. J Bacteriol. 1993 Jul;175(13):4104–4120. doi: 10.1128/jb.175.13.4104-4120.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S. L., Sanderson K. E. A physical map of the Salmonella typhimurium LT2 genome made by using XbaI analysis. J Bacteriol. 1992 Mar;174(5):1662–1672. doi: 10.1128/jb.174.5.1662-1672.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez J. G., Vela G. R. True morphology of the Azotobacteraceae-filterable bacteria. Nature. 1981 Feb 12;289(5798):588–590. doi: 10.1038/289588a0. [DOI] [PubMed] [Google Scholar]
- Løbner-Olesen A., Skarstad K., Hansen F. G., von Meyenburg K., Boye E. The DnaA protein determines the initiation mass of Escherichia coli K-12. Cell. 1989 Jun 2;57(5):881–889. doi: 10.1016/0092-8674(89)90802-7. [DOI] [PubMed] [Google Scholar]
- Maldonado R., Garzón A., Dean D. R., Casadesús J. Gene dosage analysis in Azotobacter vinelandii. Genetics. 1992 Dec;132(4):869–878. doi: 10.1093/genetics/132.4.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manna A. C., Das H. K. Determination of the size of the Azotobacter vinelandii chromosome. Mol Gen Genet. 1993 Dec;241(5-6):719–722. doi: 10.1007/BF00279916. [DOI] [PubMed] [Google Scholar]
- McClelland M., Jones R., Patel Y., Nelson M. Restriction endonucleases for pulsed field mapping of bacterial genomes. Nucleic Acids Res. 1987 Aug 11;15(15):5985–6005. doi: 10.1093/nar/15.15.5985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon A. L., Stults L. W., Robson R. L., Mortenson L. E. Cloning, sequencing and characterization of the [NiFe]hydrogenase-encoding structural genes (hoxK and hoxG) from Azotobacter vinelandii. Gene. 1990 Nov 30;96(1):67–74. doi: 10.1016/0378-1119(90)90342-o. [DOI] [PubMed] [Google Scholar]
- Merrick M., Gibbins J., Toukdarian A. The nucleotide sequence of the sigma factor gene ntrA (rpoN) of Azotobacter vinelandii: analysis of conserved sequences in NtrA proteins. Mol Gen Genet. 1987 Dec;210(2):323–330. doi: 10.1007/BF00325701. [DOI] [PubMed] [Google Scholar]
- Moshiri F., Chawla A., Maier R. J. Cloning, characterization, and expression in Escherichia coli of the genes encoding the cytochrome d oxidase complex from Azotobacter vinelandii. J Bacteriol. 1991 Oct;173(19):6230–6241. doi: 10.1128/jb.173.19.6230-6241.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagpal P., Jafri S., Reddy M. A., Das H. K. Multiple chromosomes of Azotobacter vinelandii. J Bacteriol. 1989 Jun;171(6):3133–3138. doi: 10.1128/jb.171.6.3133-3138.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paau A. S., Cowles J. R., Oro J. Flow-microfluorometric analysis of Escherichia coli, Rhizobium meliloti, and Rhizobium japonicum at different stages of the growth cycle. Can J Microbiol. 1977 Sep;23(9):1165–1169. doi: 10.1139/m77-175. [DOI] [PubMed] [Google Scholar]
- Page W. J., Cornish A. Growth of Azotobacter vinelandii UWD in Fish Peptone Medium and Simplified Extraction of Poly-beta-Hydroxybutyrate. Appl Environ Microbiol. 1993 Dec;59(12):4236–4244. doi: 10.1128/aem.59.12.4236-4244.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips G. J., Arnold J., Ivarie R. Mono- through hexanucleotide composition of the Escherichia coli genome: a Markov chain analysis. Nucleic Acids Res. 1987 Mar 25;15(6):2611–2626. doi: 10.1093/nar/15.6.2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips G. J., Arnold J., Ivarie R. The effect of codon usage on the oligonucleotide composition of the E. coli genome and identification of over- and underrepresented sequences by Markov chain analysis. Nucleic Acids Res. 1987 Mar 25;15(6):2627–2638. doi: 10.1093/nar/15.6.2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postgate J. R., Kent H. M., Robson R. L., Chesshyre J. A. The genomes of Desulfovibrio gigas and D. vulgaris. J Gen Microbiol. 1984 Jul;130(7):1597–1601. doi: 10.1099/00221287-130-7-1597. [DOI] [PubMed] [Google Scholar]
- Roberts G. P., Brill W. J. Genetics and regulation of nitrogen fixation. Annu Rev Microbiol. 1981;35:207–235. doi: 10.1146/annurev.mi.35.100181.001231. [DOI] [PubMed] [Google Scholar]
- Robson R. L., Chesshyre J. A., Wheeler C., Jones R., Woodley P. R., Postgate J. R. Genome size and complexity in Azotobacter chroococcum. J Gen Microbiol. 1984 Jul;130(7):1603–1612. doi: 10.1099/00221287-130-7-1603. [DOI] [PubMed] [Google Scholar]
- Sadoff H. L., Berke E., Loperfido B. Physiological studies of encystment in Azotobacter vinelandii. J Bacteriol. 1971 Jan;105(1):185–189. doi: 10.1128/jb.105.1.185-189.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadoff H. L., Shimel B., Ellis S. Characterization of Azotobacter vinelandii deoxyribonucleic acid and folded chromosomes. J Bacteriol. 1979 Jun;138(3):871–877. doi: 10.1128/jb.138.3.871-877.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skarstad K., Boye E., Steen H. B. Timing of initiation of chromosome replication in individual Escherichia coli cells. EMBO J. 1986 Jul;5(7):1711–1717. doi: 10.1002/j.1460-2075.1986.tb04415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skarstad K., Steen H. B., Boye E. Cell cycle parameters of slowly growing Escherichia coli B/r studied by flow cytometry. J Bacteriol. 1983 May;154(2):656–662. doi: 10.1128/jb.154.2.656-662.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skarstad K., Steen H. B., Boye E. Escherichia coli DNA distributions measured by flow cytometry and compared with theoretical computer simulations. J Bacteriol. 1985 Aug;163(2):661–668. doi: 10.1128/jb.163.2.661-668.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. L., Econome J. G., Schutt A., Klco S., Cantor C. R. A physical map of the Escherichia coli K12 genome. Science. 1987 Jun 12;236(4807):1448–1453. doi: 10.1126/science.3296194. [DOI] [PubMed] [Google Scholar]
- Steen H. B., Lindmo T. Flow cytometry: a high-resolution instrument for everyone. Science. 1979 Apr 27;204(4391):403–404. doi: 10.1126/science.441727. [DOI] [PubMed] [Google Scholar]
- Toukdarian A., Saunders G., Selman-Sosa G., Santero E., Woodley P., Kennedy C. Molecular analysis of the Azotobacter vinelandii glnA gene encoding glutamine synthetase. J Bacteriol. 1990 Nov;172(11):6529–6539. doi: 10.1128/jb.172.11.6529-6539.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- WITKIN E. M. Nuclear segregation and the delayed appearance of induced mutants in Escherichia coli. Cold Spring Harb Symp Quant Biol. 1951;16:357–372. doi: 10.1101/sqb.1951.016.01.027. [DOI] [PubMed] [Google Scholar]
- Wehrli W., Staehelin M. Actions of the rifamycins. Bacteriol Rev. 1971 Sep;35(3):290–309. doi: 10.1128/br.35.3.290-309.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westphal A. H., de Kok A. Lipoamide dehydrogenase from Azotobacter vinelandii. Molecular cloning, organization and sequence analysis of the gene. Eur J Biochem. 1988 Mar 1;172(2):299–305. doi: 10.1111/j.1432-1033.1988.tb13887.x. [DOI] [PubMed] [Google Scholar]
- Wu F. J., Moreno J., Vela G. R. Growth of Azotobacter vinelandii on Soil Nutrients. Appl Environ Microbiol. 1987 Mar;53(3):489–494. doi: 10.1128/aem.53.3.489-494.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]




