Abstract
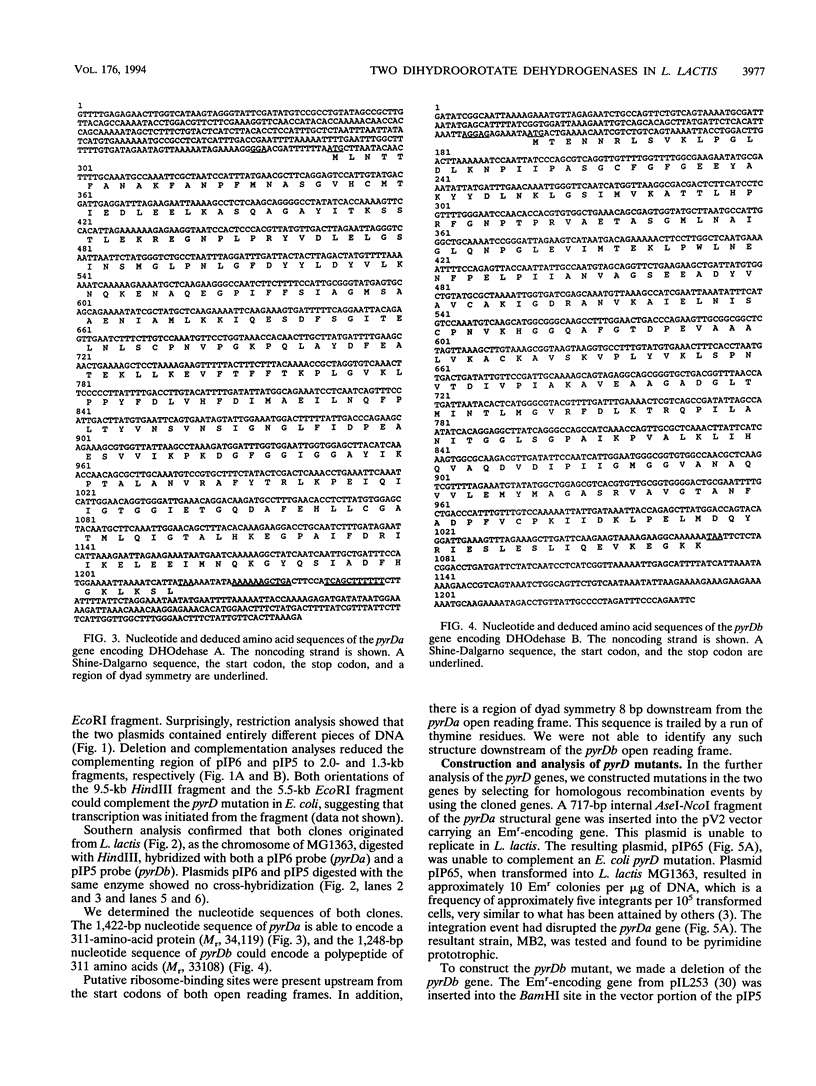
The pyrimidine de novo biosynthesis pathway has been characterized for a number of organisms. The general pathway consists of six enzymatic steps. In the characterization of the pyrimidine pathway of Lactococcus lactis, two different pyrD genes encoding dihydroorotate dehydrogenase were isolated. The nucleotide sequences of the two genes, pyrDa and pyrDb, have been determined. One of the deduced amino acid sequences has a high degree of homology to the Saccharomyces cerevisiae dihydroorotate dehydrogenase, and the other resembles the dihydroorotate dehydrogenase from Bacillus subtilis. It is possible to distinguish between the two enzymes in crude extracts by using different electron acceptors. We constructed mutants containing a mutated form of either one or the other or both of the pyrD genes. Only the double mutant is pyrimidine auxotrophic.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews S., Cox G. B., Gibson F. The anaerobic oxidation of dihydroorotate by Escherichia coli K-12. Biochim Biophys Acta. 1977 Oct 12;462(1):153–160. doi: 10.1016/0005-2728(77)90197-9. [DOI] [PubMed] [Google Scholar]
- Bardowski J., Ehrlich S. D., Chopin A. Tryptophan biosynthesis genes in Lactococcus lactis subsp. lactis. J Bacteriol. 1992 Oct;174(20):6563–6570. doi: 10.1128/jb.174.20.6563-6570.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas I., Gruss A., Ehrlich S. D., Maguin E. High-efficiency gene inactivation and replacement system for gram-positive bacteria. J Bacteriol. 1993 Jun;175(11):3628–3635. doi: 10.1128/jb.175.11.3628-3635.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delorme C., Ehrlich S. D., Renault P. Histidine biosynthesis genes in Lactococcus lactis subsp. lactis. J Bacteriol. 1992 Oct;174(20):6571–6579. doi: 10.1128/jb.174.20.6571-6579.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasson M. J. Plasmid complements of Streptococcus lactis NCDO 712 and other lactic streptococci after protoplast-induced curing. J Bacteriol. 1983 Apr;154(1):1–9. doi: 10.1128/jb.154.1.1-9.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godon J. J., Chopin M. C., Ehrlich S. D. Branched-chain amino acid biosynthesis genes in Lactococcus lactis subsp. lactis. J Bacteriol. 1992 Oct;174(20):6580–6589. doi: 10.1128/jb.174.20.6580-6589.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hines V., Keys L. D., 3rd, Johnston M. Purification and properties of the bovine liver mitochondrial dihydroorotate dehydrogenase. J Biol Chem. 1986 Aug 25;261(24):11386–11392. [PubMed] [Google Scholar]
- Holo H., Nes I. F. High-Frequency Transformation, by Electroporation, of Lactococcus lactis subsp. cremoris Grown with Glycine in Osmotically Stabilized Media. Appl Environ Microbiol. 1989 Dec;55(12):3119–3123. doi: 10.1128/aem.55.12.3119-3123.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen P. R., Hammer K. Minimal Requirements for Exponential Growth of Lactococcus lactis. Appl Environ Microbiol. 1993 Dec;59(12):4363–4366. doi: 10.1128/aem.59.12.4363-4366.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karibian D., Couchoud P. Dihydro-orotate oxidase of Escherichia coli K12: purification, properties, and relation to the cytoplasmic membrane. Biochim Biophys Acta. 1974 Oct 17;364(2):218–232. doi: 10.1016/0005-2744(74)90007-2. [DOI] [PubMed] [Google Scholar]
- Karibian D. Dihydroorotate dehydrogenase (Escherichia coli). Methods Enzymol. 1978;51:58–63. doi: 10.1016/s0076-6879(78)51010-0. [DOI] [PubMed] [Google Scholar]
- Kerr C. T., Miller R. W. Soluble NADP-linked dihydroorotate dehydrogenase from a pseudomonad. Can J Biochem. 1967 Sep;45(9):1295–1307. doi: 10.1139/o67-151. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Larsen J. N., Jensen K. F. Nucleotide sequence of the pyrD gene of Escherichia coli and characterization of the flavoprotein dihydroorotate dehydrogenase. Eur J Biochem. 1985 Aug 15;151(1):59–65. doi: 10.1111/j.1432-1033.1985.tb09068.x. [DOI] [PubMed] [Google Scholar]
- Llanos R. M., Harris C. J., Hillier A. J., Davidson B. E. Identification of a novel operon in Lactococcus lactis encoding three enzymes for lactic acid synthesis: phosphofructokinase, pyruvate kinase, and lactate dehydrogenase. J Bacteriol. 1993 May;175(9):2541–2551. doi: 10.1128/jb.175.9.2541-2551.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. W. Dihydroorotate dehydrogenase (Neurospora). Methods Enzymol. 1978;51:63–69. doi: 10.1016/s0076-6879(78)51011-2. [DOI] [PubMed] [Google Scholar]
- Miller R. W., Kerr C. T. Particulate dihydroorotate oxidase system from a pseudomonad. Linkage with the respiratory chain. Can J Biochem. 1967 Sep;45(9):1283–1294. doi: 10.1139/o67-150. [DOI] [PubMed] [Google Scholar]
- Nagy M., Lacroute F., Thomas D. Divergent evolution of pyrimidine biosynthesis between anaerobic and aerobic yeasts. Proc Natl Acad Sci U S A. 1992 Oct 1;89(19):8966–8970. doi: 10.1073/pnas.89.19.8966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton N. A., Cox G. B., Gibson F. The function of menaquinone (vitamin K 2 ) in Escherichia coli K-12. Biochim Biophys Acta. 1971 Jul 20;244(1):155–166. doi: 10.1016/0304-4165(71)90132-2. [DOI] [PubMed] [Google Scholar]
- Nilsson B., Uhlén M., Josephson S., Gatenbeck S., Philipson L. An improved positive selection plasmid vector constructed by oligonucleotide mediated mutagenesis. Nucleic Acids Res. 1983 Nov 25;11(22):8019–8030. doi: 10.1093/nar/11.22.8019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson D., Lauridsen A. A. Isolation of purine auxotrophic mutants of Lactococcus lactis and characterization of the gene hpt encoding hypoxanthine guanine phosphoribosyltransferase. Mol Gen Genet. 1992 Nov;235(2-3):359–364. doi: 10.1007/BF00279381. [DOI] [PubMed] [Google Scholar]
- Pascal R. A., Jr, Le Trang N., Cerami A., Walsh C. Purification and properties of dihydroorotate oxidase from Crithidia fasciculata and Trypanosoma brucei. Biochemistry. 1983 Jan 4;22(1):171–178. doi: 10.1021/bi00270a025. [DOI] [PubMed] [Google Scholar]
- Quinn C. L., Stephenson B. T., Switzer R. L. Functional organization and nucleotide sequence of the Bacillus subtilis pyrimidine biosynthetic operon. J Biol Chem. 1991 May 15;266(14):9113–9127. [PubMed] [Google Scholar]
- Roy A. Nucleotide sequence of the URA1 gene of Saccharomyces cerevisiae. Gene. 1992 Sep 1;118(1):149–150. doi: 10.1016/0378-1119(92)90265-q. [DOI] [PubMed] [Google Scholar]
- Simon D., Chopin A. Construction of a vector plasmid family and its use for molecular cloning in Streptococcus lactis. Biochimie. 1988 Apr;70(4):559–566. doi: 10.1016/0300-9084(88)90093-4. [DOI] [PubMed] [Google Scholar]
- Taylor M. L., Taylor W. H., Eames D. F., Taylor C. D. Biosynthetic dihydroorotate dehydrogenase from Lactobacillus bulgaricus. J Bacteriol. 1971 Mar;105(3):1015–1027. doi: 10.1128/jb.105.3.1015-1027.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor W. H., Taylor C. D., Taylor M. L. Biosynthetic dihydroorotate dehydrogenase from Lactobacillus bulgaricus: partial characterization of the enzyme. J Bacteriol. 1974 Jul;119(1):98–105. doi: 10.1128/jb.119.1.98-105.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor W. H., Taylor M. L., Eames D. F. Two functionally different dihydroorotic dehydrogenases in bacteria. J Bacteriol. 1966 Jun;91(6):2251–2256. doi: 10.1128/jb.91.6.2251-2256.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogels G. D., Van der Drift C. Degradation of purines and pyrimidines by microorganisms. Bacteriol Rev. 1976 Jun;40(2):403–468. doi: 10.1128/br.40.2.403-468.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisblum B., Graham M. Y., Gryczan T., Dubnau D. Plasmid copy number control: isolation and characterization of high-copy-number mutants of plasmid pE194. J Bacteriol. 1979 Jan;137(1):635–643. doi: 10.1128/jb.137.1.635-643.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]




