Abstract
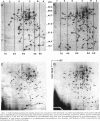
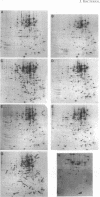
Pseudomonas fragi has the ability to grow between 0 and 35 degrees C and grows optimally at 30 degrees C. Cellular proteins from mid-log-phase cells growing from 4 to 34 degrees C were labeled with L-[35S]methionine during 1 generation time and analyzed by two-dimensional gel electrophoresis. The electrophoretic patterns revealed differences in the patterns of protein synthesis over this temperature span. A qualitative comparison of cellular proteins led to their separation into five thermal classes. The first class contained proteins whose relative rates of synthesis were unaffected by the growth temperature. Three other classes included proteins with optimal expression at 4 to 10, 15 to 20, and 25 to 30 degrees C. A fifth class contained proteins which were more specifically synthesized at a supraoptimal growth temperature (34 degrees C). Two low-molecular-mass proteins, designated C7.0 and C8.0, were highly concentrated at 4 to 10 degrees C, and their relative rates of synthesis steadily increased with decreasing temperature. Polyclonal antibodies were separately raised against these two proteins. Immunological analyses revealed cross-reaction between these two proteins and between two additional low-molecular-mass proteins which were maximally produced at elevated temperatures. Antisera directed against C8.0 recognized the major cold shock protein of Escherichia coli, CspA, indicating the presence of similarities between these proteins.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Araki T. Changes in rates of synthesis of individual proteins in a psychrophilic bacterium after a shift in temperature. Can J Microbiol. 1991 Nov;37(11):840–847. doi: 10.1139/m91-145. [DOI] [PubMed] [Google Scholar]
- Araki T. The effect of temperature shifts on protein synthesis by the psychrophilic bacterium Vibrio sp. strain ANT-300. J Gen Microbiol. 1991 Apr;137(4):817–826. doi: 10.1099/00221287-137-4-817. [DOI] [PubMed] [Google Scholar]
- Av-Gay Y., Aharonowitz Y., Cohen G. Streptomyces contain a 7.0 kDa cold shock like protein. Nucleic Acids Res. 1992 Oct 25;20(20):5478–5478. doi: 10.1093/nar/20.20.5478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Broeze R. J., Solomon C. J., Pope D. H. Effects of low temperature on in vivo and in vitro protein synthesis in Escherichia coli and Pseudomonas fluorescens. J Bacteriol. 1978 Jun;134(3):861–874. doi: 10.1128/jb.134.3.861-874.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dainty R. H., Shaw B. G., Roberts T. A. Microbial and chemical changes in chill-stored red meats. Soc Appl Bacteriol Symp Ser. 1983;11:151–178. [PubMed] [Google Scholar]
- Fairbairn D. J., Law B. A. Proteinases of psychrotrophic bacteria: their production, properties, effects and control. J Dairy Res. 1986 Feb;53(1):139–177. doi: 10.1017/s0022029900024742. [DOI] [PubMed] [Google Scholar]
- Goldstein J., Pollitt N. S., Inouye M. Major cold shock protein of Escherichia coli. Proc Natl Acad Sci U S A. 1990 Jan;87(1):283–287. doi: 10.1073/pnas.87.1.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gounot A. M. Bacterial life at low temperature: physiological aspects and biotechnological implications. J Appl Bacteriol. 1991 Nov;71(5):386–397. doi: 10.1111/j.1365-2672.1991.tb03806.x. [DOI] [PubMed] [Google Scholar]
- Granston A. E., Thompson D. L., Friedman D. I. Identification of a second promoter for the metY-nusA-infB operon of Escherichia coli. J Bacteriol. 1990 May;172(5):2336–2342. doi: 10.1128/jb.172.5.2336-2342.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gügi B., Orange N., Hellio F., Burini J. F., Guillou C., Leriche F., Guespin-Michel J. F. Effect of growth temperature on several exported enzyme activities in the psychrotrophic bacterium Pseudomonas fluorescens. J Bacteriol. 1991 Jun;173(12):3814–3820. doi: 10.1128/jb.173.12.3814-3820.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herendeen S. L., VanBogelen R. A., Neidhardt F. C. Levels of major proteins of Escherichia coli during growth at different temperatures. J Bacteriol. 1979 Jul;139(1):185–194. doi: 10.1128/jb.139.1.185-194.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horii T., Ogawa T., Ogawa H. Organization of the recA gene of Escherichia coli. Proc Natl Acad Sci U S A. 1980 Jan;77(1):313–317. doi: 10.1073/pnas.77.1.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaenicke R. Protein structure and function at low temperatures. Philos Trans R Soc Lond B Biol Sci. 1990 Jan 30;326(1237):535–553. doi: 10.1098/rstb.1990.0030. [DOI] [PubMed] [Google Scholar]
- Jones P. G., Cashel M., Glaser G., Neidhardt F. C. Function of a relaxed-like state following temperature downshifts in Escherichia coli. J Bacteriol. 1992 Jun;174(12):3903–3914. doi: 10.1128/jb.174.12.3903-3914.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones P. G., Krah R., Tafuri S. R., Wolffe A. P. DNA gyrase, CS7.4, and the cold shock response in Escherichia coli. J Bacteriol. 1992 Sep;174(18):5798–5802. doi: 10.1128/jb.174.18.5798-5802.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Teana A., Brandi A., Falconi M., Spurio R., Pon C. L., Gualerzi C. O. Identification of a cold shock transcriptional enhancer of the Escherichia coli gene encoding nucleoid protein H-NS. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10907–10911. doi: 10.1073/pnas.88.23.10907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- McKellar R. C. Factors influencing the production of extracellular proteinase by Pseudomonas fluorescens. J Appl Bacteriol. 1982 Dec;53(3):305–316. doi: 10.1111/j.1365-2672.1982.tb01276.x. [DOI] [PubMed] [Google Scholar]
- Morita R. Y. Psychrophilic bacteria. Bacteriol Rev. 1975 Jun;39(2):144–167. doi: 10.1128/br.39.2.144-167.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Qoronfleh M. W., Debouck C., Keller J. Identification and characterization of novel low-temperature-inducible promoters of Escherichia coli. J Bacteriol. 1992 Dec;174(24):7902–7909. doi: 10.1128/jb.174.24.7902-7909.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell N. J. Cold adaptation of microorganisms. Philos Trans R Soc Lond B Biol Sci. 1990 Jan 30;326(1237):595-608, discussion 608-11. doi: 10.1098/rstb.1990.0034. [DOI] [PubMed] [Google Scholar]
- Szer W. Cell-free protein synthesis at 0 degrees. An activating factor from ribosomes of a psychrophilic microorganism. Biochim Biophys Acta. 1970 Jul 16;213(1):159–170. [PubMed] [Google Scholar]
- VanBogelen R. A., Neidhardt F. C. The gene-protein database of Escherichia coli: edition 4. Electrophoresis. 1991 Nov;12(11):955–994. doi: 10.1002/elps.1150121114. [DOI] [PubMed] [Google Scholar]
- Willimsky G., Bang H., Fischer G., Marahiel M. A. Characterization of cspB, a Bacillus subtilis inducible cold shock gene affecting cell viability at low temperatures. J Bacteriol. 1992 Oct;174(20):6326–6335. doi: 10.1128/jb.174.20.6326-6335.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolffe A. P., Tafuri S., Ranjan M., Familari M. The Y-box factors: a family of nucleic acid binding proteins conserved from Escherichia coli to man. New Biol. 1992 Apr;4(4):290–298. [PubMed] [Google Scholar]