Abstract
The perturbation of various glycosylphosphatidylinositol (GPI)-anchored surface proteins imparts profound regulatory signals to macrophages, lymphocytes and other cell types. The specific contribution of the GPI moieties to these events however is unclear. This study demonstrates that purified GPIs of Plasmodium falciparum, Trypanosoma brucei, and Leishmania mexicana origin are sufficient to initiate signal transduction when added alone to host cells as chemically defined agonists. GPIs (10 nM–1 μM) induce rapid activation of the protein tyrosine kinase (PTK) p59hck in macrophages. The minimal structural requirement for PTK activation is the evolutionarily conserved core glycan sequence Manα1-2Manα1-6Manα1-4GlcN1-6myo-inositol. GPI-associated diacylglycerols independently activate the calcium-independent ɛ isoform of protein kinase C. Both signals collaborate in regulating the downstream NF-κB/rel-dependent gene expression of interleukin 1α, tumor necrosis factor (TNF) α, and inducible NO synthase. The alkylacylglycerol-containing iM4 GIPL of L. mexicana, however, is unable to activate protein kinase C and inhibits TNF expression in response to other agonists, establishing signaling specificity among structurally distinct GPIs. GPI alone appears sufficient to mimic the activities of malaria parasite extracts in the signaling pathway leading to TNF expression. A mAb to GPI blocks TNF induction by parasite extracts indicating that GPI is a necessary agent in this response. As protozoal GPIs are closely related to their mammalian counterparts, the data indicate that GPIs do indeed constitute a novel outside-in signaling system, acting as both agonists and second messenger substrates, and imparting at least two separate signals through the structurally distinct glycan and fatty acid domains. These activities may underlie aspects of pathology and immune regulation in protozoal infections.
Glycosylphosphatidylinositols (GPIs) are a class of glycolipid common to all eukaryotes, first described from Trypanosoma brucei (1), and characterized since from many protozoal and mammalian sources (2). Recent evidence suggests GPIs may mediate signal transduction within cells. Crosslinking various GPI-anchored surface proteins imparts profound regulatory signals to T lymphocytes (3, 4). In macrophages and neutrophils, crosslinking GPI-anchored CD14 [lipopolysaccharide (LPS) receptor] and CD16 (Fc-γRIIIB) induces activation leading to cytokine expression and oxidative burst (5, 6). GPI-anchored proteins are physically associated with the src-family protein tyrosine kinases (PTKs) fyn, lck (7–9), fgr, lyn, and hck (10), and crosslinking GPI-anchored proteins induces PTK-mediated tyrosylphosphorylation. GPIs may also act as precursors for autocrine/paracrine diffusable second messengers in postreceptor signaling in response to various hormones and cytokines (11–14), which reportedly induce the hydrolysis of GPI-like molecules and the release of pharmacologically active inositolphosphoglycans (IPGs). Nonetheless, much of this data remains circumstantial and indirect. Antibody-mediated crosslinking of GPI-anchored proteins, often in the presence of phorbol esters, is of unknown physiological relevance and cannot always distinguish between a contribution by the protein component, the purely physical properties of the GPI moieties (lateral diffusion), artifactual disruption of other signaling components (e.g., the T cell antigen receptor complex), and GPI-mediated signaling per se. Furthermore the mechanism of signal transduction by GPI remains unclear, and a topological objection remains: since GPI-anchored proteins are in the outer (luminal) leaflet of the plasma membrane, how is a GPI-mediated activation signal transmitted across the membrane to the interior of the cell? Finally, no structure of any hormone/cytokine-sensitive mammalian GPI has yet been determined, and the description of these putative second messengers as GPI-derived IPGs remains speculative. Indeed the biological activities ascribed to IPG fragments cannot yet be attributed to pure, structurally defined moieties.
To confirm then that GPIs can act as signaling agents, it should be sufficient to obtain specific signaling events by the addition of purified GPIs alone to cells as defined agonists. We have shown that GPIs of Plasmodium and Trypanosoma induce the expression in host cells of inducible NO synthase (iNOS), tumor necrosis factor (TNF), IL-1, and adhesins (15–18), and can induce lethal cachexia in vivo (15). Others have shown that Leishmania glycoinositolphospholipid (GIPLs) inhibit NO output (19). As these host responses are implicated both in malarial and trypanosomal pathophysiology (20, 21) and survival of Leishmania (19), GPI/GIPLs are candidate pathogenicity factors, but their mechanism of action remains unclear. Our objectives in this study thus were two-fold: (i) as protozoal GPIs are closely related to mammalian GPIs, to determine whether structurally distinct protozoal GPIs might directly activate signaling pathways in host cells, and thereby validate the proposed role of GPIs as novel signaling mechanisms; and (ii) to elucidate further the molecular basis for GPI toxicity, with a view to providing novel targets for the therapy of severe malaria infection. Our findings demonstrate that GPIs act as both agonists and second messenger substrates in initiating novel outside-in signaling pathways, and point to their use as probes for the further investigation of GPI-based signaling. The data furthermore demonstrate specificity of action among structurally distinct GPIs from various protozoal taxa, suggesting that these molecules serve differentially to regulate host cell function in protozoal infections.
MATERIALS AND METHODS
Purification of the C-terminal GPI Anchors of Defined Parasite antigens.
GPI-anchored Plasmodium falciparum MSP-1, MSP-2, and Trypanosoma brucei 118 (MITat 1.5) membrane-form variant surface glycoprotein (mfVSG) were fatty-acid labeled and purified as previously described (15–17), to 10 mg/ml. Methanol (600 μl) was added to 150 μl aliquots followed by 150 μl CHCl3 and 450 μl H2O. The samples were vortexed and microfuged, the supernatant taken for scintillation counting, and the interphase and lower phase mixed with 450 μl methanol and recentrifuged. The pellet was repeatedly extracted with chloroform/methanol/water (CMW; 10:10:3) until partitioning of label into the supernatant was minimal, partitioned between water and water-saturated butanol, precipitated with acetone at −20°C, and the proteins taken up by sonication in 500 μl 100 mM Tris·HCl/5 mM CaCl2. Predigested Pronase B (2.5%) was added, and incubated for 72 h at 37°C with two additions of 0.25% pronase. The sample was loaded in 5% 1-propanol/0.1 M NH4OAc onto preequilibrated Octyl-Sepharose, washed and eluted in a linear gradient of 1-propanol (5–60%) in water. GPIs were eluted at 35–40% 1-propanol and were spotted onto TLC plates (Si-60) and run in the solvent system C/M/HAc/W 25:15:4:2. The origin was scraped, and GPIs were eluted and partitioned between water and water-saturated butanol.
Purification of GIPLs and GPI Precursors by TLC.
[H]-glucosamine-labeled P. falciparum schizonts (2 × 1010) were extracted in chloroform/methanol (CM; 2:1) and CMW (1:1:0.3), Folch washed, partitioned between water and water-saturated butanol, and dried. Residues were separated by two-dimensional (2D) TLC (first dimension CMW 4:4:1, second dimension Butanol/HAc/W 4:6:1), the plates scanned by Bertold Digital Autoradiograph scanner, and the structurally defined (22, 23) GPI peaks scraped and reextracted. Phospholipids were resolved away from GPI peaks. Areas lying outside the identifiable GPI peaks were treated in the same way, as were sham plates. GIPLs of Leishmania mexicana were purified to homogeneity as described (24, 25). Briefly, promastigotes were extracted twice in CMW (1:2:0.8), the insoluble material removed by centrifugation, and the CMW phase partitioned with 0.6 volume water. The dried upper phase was chromatographed on Octyl-Sepharose as above, eluted GIPLs were further purified by high-performance TLC using CM/1 M NH4OH (10:10:3), and scrapings were extracted with CMW (1:2:0.8). GIPL concentration and compositional purity was determined by GC-MS (24, 25), following acid methanolysis and trimethylsilyl derivatization. myo-Inositol content was measured following acid hydrolysis (6 M HCl at 110°C for 16 h) and trimethylsilyl derivatization, with selected ion monitoring for m/z 305 and 308. Scyllo-inositol was used as internal standard throughout.
Generation of Chemical and Enzymatic Hydrolysis Fragments of GPIs.
GPIs were base hydrolyzed in methanol/ammonia 1:1 for 6 h at 50°C, and partioned between water and water-saturated butanol. The aqueous phase was twice extracted with water-saturated butanol, dried, and repeatedly flash evaporated with methanol. For enzymatic hydrolysis of GPIs, the TLC-purified glycolipids were dried and redissolved in 0.1 mM Tris·HCl/2 mM CaCl2/30 mM N-octylthioglucopyranoside, pH 7.4. GPI-specific phospholipase D (GPI-PLD) or PLA2 (Boehringer Mannheim) were added at 37°C for 3 h or overnight, respectively. The reaction products were separated by TLC in CMW 4:4:1. The resolved hydrolysis fragments were detected by Berthold digital autoradiograph and recovered by scraping the plates.
Culture and Activation of Macrophages.
RAW 264 cells were maintained in RPMI medium 1640 with 10% fetal calf serum. LPS-nonresponsive C3H/HeJ macrophages were obtained as described (15, 18). Adherent cells (2 × 105 cells per well) were given medium alone or test agents. Three hours after incubation TNF-α levels in the supernatant and standard curve were determined by capture ELISA (PharMingen). TNF-α and IL-1α gene expression was detected by reverse transcription–PCR using standard protocols.
Determination of Intracellular Activation of Protein Kinase C (PKC) and Tyrosine Phosphorylation.
Cells were incubated at 37°C for 30 min with agonists, resuspended in 0.5 M sucrose, 0.02% leupeptin, 2 mM phenylmethylsulfonyl fluoride, 20 mM 2-mercaptoethanol, 4 mM EDTA, and 1 mM EGTA (pH 7.5), sonicated, centrifuged at 100,000 × g for 60 min at 4°C, and the cytosolic fraction collected. The pellet was sonicated in the same buffer with 1% Triton X-100 and centrifuged for 10 min at 14,000 × g at 4°C to obtain the supernatant (membrane fraction). Equal amounts of cytosolic or membrane protein were subjected to SDS/PAGE and Western blot analysis with mAbs against the α, β, δ, ɛ, and θ PKC isoenzymes (Transduction Laboratories), and detected by enhanced chemiluminescence (Amersham). Tyrosine phosphorylation of the TX-100 soluble lysate was determined as previously described (16–18).
Immunoprecipitation and in Vitro Kinase Assays.
Cells were lysed in 50 mM Tris·HCl, pH 7.5/150 mM NaCl/2 mM EDTA/1% Nonidet P-40/0.1% Tween 20/0.1% SDS/10 μg/ml aprotinin/10 μg/ml leupeptin/5 mM NaF/1 mM phenylmethylsulfonyl fluoride/4 mM tosyl-l-lysine chloromethyl ketone/4 mM Na3VO4 (1 h on ice), precleared, specifically immunoprecipitated with polyclonal antikinase antisera and protein A-Sepharose, washed, mixed with 20 μl kinase assay buffer (20 mM Mops, pH 7.0/5 mM MnCl2), 2.5 μl poly(Glu, Tyr) 4:1 (2.5 mg/ml) or acid-denatured enolase, 2.5 μl [γ-33P]ATP [3,000 Ci/mmol (1 Ci = 37 GBq)], and incubated for 30 min at room temperature. Samples were subjected to SDS/PAGE and fluorography or Western blot analysis.
RESULTS
Purification of Parasite GPIs and GIPLs.
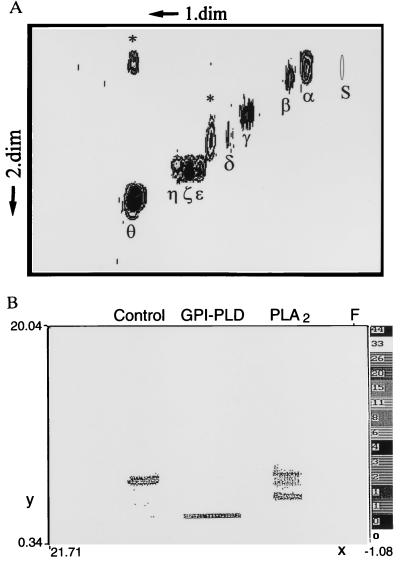
For this study a number of complementary purification protocols were employed. Two-dimensional TLC revealed the structurally defined P. falciparum GPI precursors Pfglα-Pfglθ, following the nomenclature of Gerold et al. (22) (Fig. 1A). Phospholipase hydrolysis products of Pfglpα were obtained as shown in Fig. 1B. Compositional analysis on P. falciparum GPI precursors purified by TLC showed some minor batch-to-batch variability in contamination with lipids (data not shown). Therefore we utilized in addition both L. mexicana GIPLs (nomenclature as in refs. 2, 24, and 25) and mature GPIs, purified after extensive organic solvent extraction of proteins to remove noncovalently associated lipids and phospholipids, followed by pronase digestion, Octyl-Sepharose chromatography, and TLC. The compositional purity of the GIPLs was confirmed by GC-MS as described (24, 25).
Figure 1.
Purification of GPI precursors and hydrolysis fragments by TLC. (A) 2D TLC of P. falciparum [3H]-glucosamine-labeled GPI precursors (22, 23). Asterisks indicate uncharacterized glycolipids. (B) TLC-purified GPI (Pfgl-α, Rf 0.2) was hydrolyzed enzymatically and the products resolved by 1D TLC in CMW 4:4:1 [GPI-PLD yielding 100% conversion to IPG, Rf 0.01; PLA2 yielding 35% conversion to lyso-GPI, as previously reported (22), Rf 0.1].
GPI Stimulates p59hck and Tyrosine Phosphorylation of Multiple Substrates in Macrophages.
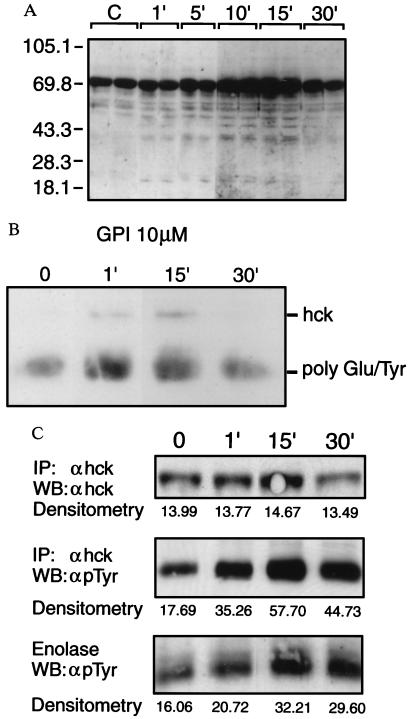
GPI-anchored proteins associate with src-family PTKs and crosslinking causes rapid onset tyrosine phosphorylation (7–10). To test the hypothesis that GPIs alone are sufficient to initiate cell signaling by activating PTKs, macrophages were treated with purified GPIs and phosphotyrosyl proteins detected by Western blot analysis. We have previously shown that T. brucei and P. falciparum GPIs are active in host cells over a concentration range of 1nM–1 μM (15–18). Therefore, in this study we used GPIs at and above the highest end of this range (1–10 μM). GPI-treated cells showed de novo phosphorylation of intracellular substrates with apparent molecular mass of 20, 22, 41, 45, 54, 56, and 58 kDa, and a marked increase in the level of a constitutively phosphorylated 64- to 69-kDa component. Additional bands were occasionally detected with a molecular mass of 76, 85, and 95 kDa (Fig. 2A). Phosphorylation appeared by 0.5–1 min, peaked at 10–15 min, and declined after 30 min. The pattern and kinetics of tyrosylphosphorylation obtained with GPI was indistinguishable from that obtained in response to crude P. falciparum parasite extracts, suggesting that GPI is sufficient to activate the tyrosine kinase(s) responding to parasites (data not shown). To identify the PTK(s) involved in GPI signaling, in vitro kinase assays were performed on immunoprecipitates. Fig. 2 B and C show by independent methods a rapid increase in autophosphorylation of the PTK p59hck, and a significant increase above background in the transphosphorylation of two exogenous substrates, demonstrating the time-dependent activation of the enzyme. No autophosphorylation or transphosphorylation above background of exogenous substrates was observed in response to precipitation with preimmune sera.
Figure 2.
Time course of GPI-induced tyrosylphosphorylation and identification of the PTK. (A) LPS-nonresponsive macrophages were treated with GPI for the indicated times and phosphotyrosyl proteins detected by Western blot analysis. (B) In vitro kinase assay. Cells were incubated with 10 μM GPI and the reaction was stopped at various times. Lysates were precleared with rabbit preimmune serum, followed by immunoprecipitation with specific antisera to p59hck, and the immune complexes were subjected to in vitro kinase assay with exogenous ATP-γ 33P and polyGlu/Tyr as substrate, followed by SDS/PAGE and fluorography. (C) Independent experiment conducted as in B but using 1 μM GPI, cold ATP, acid-denatured enolase as exogenous substrate, and detection of phosphotyrosine or hck by Western blot analysis (WB).
The Evolutionarily Conserved GPI Core Glycan Sequence Manα1-2Manα1-6Manα1-4GlcN-myoinositol is Necessary and Sufficient to Activate PTKs.
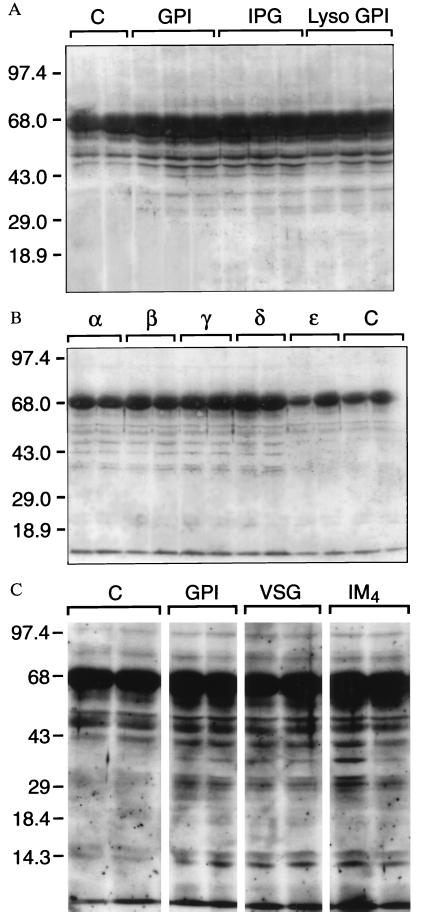
Purified GPIs were subject to chemical or enzymatic treatments to generate specific hydrolysis fragments, and the products purified away from parent material by TLC (Fig. 1B). Both PLA2-generated lyso-GPI and GPI-PLD-generated hydrophilic inositolglycan activated PTKs to a similar degree as intact GPI (Fig. 3A), indicating that fatty acids are not required for PTK activation and that the polar head group alone is sufficient for this. Malarial GPI precursors Pfglα to Pfglδ varying in their degree of glycosylation were able to activate PTKs, but Pfglɛ was not, demonstrating a requirement for the intact glycan sequence Man3-GlcN-inositol (Fig. 3B). Using comparable structures from a different source, preliminary evidence indicates that PTK activation was maximally induced by the iM4 GIPL of L. mexicana, but not precursors lacking the full glycan structure (data not shown). The single mannose substitutions to the terminal and proximal mannose residues in the core glycans of P. falciparum GPI and iM4 GIPL of L. mexicana respectively, did not influence PTK activation (Fig. 3C).
Figure 3.
The evolutionarily conserved GPI glycan is necessary and sufficient to activate PTKs. (A) Intact GPI, GPI-PLD-generated IPG, and PLA2-generated lyso-GPI activate PTK as detected by phosphotyrosine Western blot analysis. (B) Activation of PTK was observed for equal concentrations of precursor GPIs Pfglα, Pfglβ, Pfglγ, and Pfglδ, but not Pfglɛ. (C) PTK is activated equally by the T. brucei GPI (simple glycan) and the P. falciparum GPI and iM4 GIPL of L. mexicana with minor additional mannose substitutions.
GPI-Associated Diacylglycerol Is Required for Maximal Downstream Gene Expression.
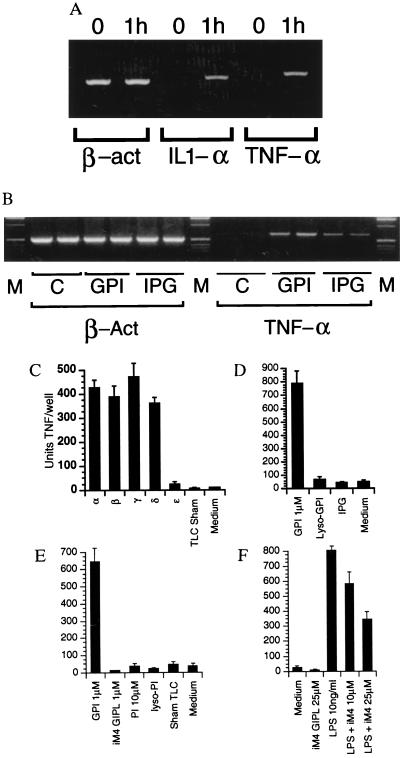
We have previously shown that malarial and trypanosomal GPIs induce TNF and IL-1 output from macrophages (15, 16). Confirming that this represents de novo gene expression, reverse transcription–PCR detects the appearance of mRNA within 1 h of addition of GPI (Fig. 4A). The ability to induce TNF of GPI precursors differing in their degree of glycosylation was then analyzed. Consistent with their ability to activate PTKs, Pfgl-α, β, γ, and δ induced TNF, but ɛ, lacking the third mannose residue, did not (Fig. 4C). The other glycolipid peaks (ζ, ν, and θ; data not shown), sham-treated TLC plates, phosphatidylinositol (PI), and lyso-PI (Sigma P2517, P0639, and L7635) also had no effect (Fig. 4E). In contrast to their effect on PTK activation, however, the GPI-PLD generated inositolglycan and PLA2-generated lyso-GPI were not sufficient for TNF induction (Fig. 4D), indicating that the GPI-associated fatty acids were required for maximal downstream gene expression. This result was confirmed for the de novo expression of TNF mRNA by reverse transcription–PCR (Fig. 4B). The iM4 GIPL was unable to induce TNF output (Fig. 4E), and substantially inhibited the TNF response to LPS (Fig. 4F). These data demonstrate a requirement for diacylglycerols in contrast to alkylacylglycerols for GPI-induced gene expression, and support the proposition (19) that GIPL alkylacylglycerols antagonize macrophage activation in response to other agonists.
Figure 4.
GPI-associated diacylglycerol is required for maximal downstream gene expression. (A) Reverse transcription–PCR confirms that GPIs induce de novo expression of TNF-α and IL-1α in macrophages. (B) de novo TNF expression is reduced in response to inositolglycan, indicating that the GPI-associated fatty acids are required for maximal downstream gene expression. (C) Induction of TNF output by GPI precursors. Macrophages were pulsed with 1 μM glycolipids Pfglα, β, γ, δ, and ɛ for 2 h and the level of TNF determined by capture ELISA. The other glycolipid peaks (ζ, ν, and θ; data not shown) and sham-treated TLC plates had no effect. (D) Inositolglycan (10 μm) and lyso-GPI are unable to induce TNF output. (E) iM4 GIPL, PI, and lyso-PI (10 μm) are unable to induce TNF output. (F) iM4 inhibits LPS-induced TNF output in RAW 264 cells.
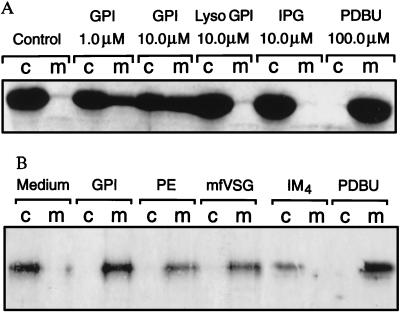
Activation of PKC by GPI Diacylglycerols.
As intact GPIs are required for TNF expression, we tested the hypothesis that GPI-associated diacylglycerols induce the activation of PKC. Macrophages were stimulated with either medium, phorbol 12,13-dibutyrate (PDBu), GPI, lyso-GPI, or IPG, and the translocation of PKC from cytosol to membrane was determined by Western blot analysis. In cells exposed to medium alone, PKC remained in the cytosol, whereas complete translocation to the membrane was observed in response to phorbol ester. Of the six PKC isoforms present in macrophages, GPI mobilized only the calcium-independent nPKCɛ (Fig. 5A), and not the other isoforms (not shown). In contrast to the activation of PTKs, IPG and lyso-GPI lost the ability to activate PKC (Fig. 5A). Both mfVSG-GPI and crude malaria parasite extracts were also able to activate PKCɛ (Fig. 5B), but not the other isoforms (not shown). Moreover, the alkylacylglycerol-containing iM4 GIPL also was unable to activate PKC (Fig. 5B). Thus GPI-associated diacylglycerols are required for PKC mobilization, which is dissociable from glycan-induced PTK activation.
Figure 5.
Activation of PKC by GPI-associated diacylglycerols. (A) Macrophages were stimulated with medium, PDBu, GPI, lyso-GPI, or IPG, and the cytosol-to-membrane translocation of nPKCɛ determined by Western blot analysis. (B) Activation of nPKCɛ by mfVSG-GPI, malarial GPI, and crude malaria parasite extracts (PE), but not the alkylacylglycerol-containing iM4 GIPL of L. mexicana. Other PKC isoforms in these cells were unaffected by all agonists except PDBu (not shown).
PTKs and PKC Cooperate and Are Required for GPI and Parasite-Induced Gene Expression.
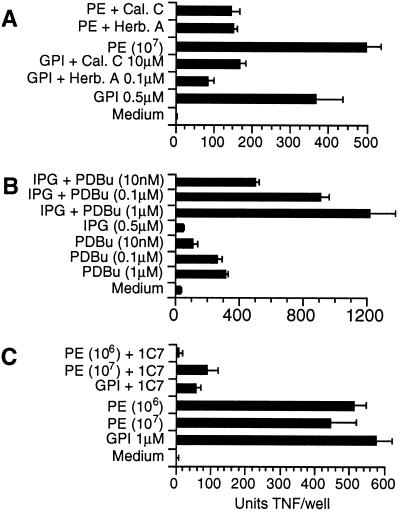
The PTK and PKC-specific antagonists herbimycin A and calphostin C at low concentrations substantially inhibited the TNF response to GPI and whole parasite extracts, indicating that both PTK and PKC are required for GPI and parasite-mediated macrophage activation (Fig. 6A). We have previously shown that the PTK-specific antagonist herbimycin A inhibits GPI-mediated tyrosine phosphorylation (17, 18). To test further the hypothesis that the glycan and diacylglycerol signals cooperate in regulation of downstream gene expression, the ability of IPG and PDBu to induce TNF output was examined. The two signals synergized, together producing significantly greater TNF output than the sum of each acting alone (Fig. 6B). As shown previously (17, 18, 26), the ability of crude P. falciparum extracts to induce TNF was blocked by a monoclonal antibody to GPI produced in this laboratory (Fig. 6C). Thus GPI is a necessary component in parasite-mediated TNF output.
Figure 6.
(A) Activation of both PKC and PTK by GPI and parasite extracts is required for downstream gene expression. The PTK and PKC-specific antagonists herbimycin A (Herb.A) and calphostin C (Cal.C) were able at 0.1 μM and 1 μM, respectively, to inhibit substantially TNF output in response to GPI and whole parasite extracts (PE). (B) IPG cooperates with PDBu in downstream TNF expression. (C) TNF output in response to crude parasites extracts (PE) and GPI is inhibited by 5 μg/ml antiGPI monoclonal 1C7.
DISCUSSION
Although GPIs are implicated in cell signaling in response to hormones (11), cytokines (12–14), and the perturbation of GPI-anchored proteins (3–10), there is no proof that GPIs constitute a signaling mechanism, and as yet no studies elucidating signaling pathways activated by GPIs acting as exogenous agonists. As malaria and trypanosome GPIs induce macrophage and endothelial cell activation (15–18), and Leishmania GIPLs have been shown to block macrophage NO output in response to IFN-γ (19), we purified GPIs from various sources for use as probes of GPI-mediated signaling.
iM4 GIPL, P. falciparum, and mfVSG-GPIs in the nM to μM range intiated rapid onset tyrosine phosphorylation in macrophages. The responding kinase was p59hck as shown by in vitro kinase assay (Fig. 2B). Hck is particularly implicated in the regulation of TNF expression as overexpression potentiates the TNF response to agonists and blockade of expression inhibits TNF synthesis (27). GPI-associated fatty acids were not required for PTK activity as tyrosine phosphorylation could be induced equally by intact GPIs/GIPLs of all three protozoal species, lyso-GPI, and IPG, suggesting that a glycan component was sufficient for this activity (Fig. 3 A and C). The P. falciparum GPI precursors (22) α, β, γ, and δ were all able to activate PTK, but ɛ was not, demonstrating a requirement for the third mannose for activity (Fig. 3B). The neutral core glycan Man3-anhydromannitol also was unable to signal (data not shown), indicating the glycan ligand lies within the sequence Manα1-2Manα1-6Manα1-4GlcN-myoinositol. Minor side-chain additions to the core glycan, as in the iM4 GIPL and P. falciparum GPI, do not appear to inhibit binding to the putative glycan-specific cell surface receptor. It remains to be determined whether more extensive modifications to the glycan will affect PTK activation.
Although sufficient to activate PTKs, lyso-GPI and IPG were less able to induce TNF output (Fig. 4 B and D), suggesting that fatty acids provide an additional signal required for maximal downstream gene expression. Accordingly, intact GPIs activated nPKCɛ, but lyso-GPI and IPG did not (Fig. 5A). Furthermore, the alkylacylglycerol-containing iM4 GIPL was also unable to activate PKC (Fig. 5B). The same structure did not induce TNF (Fig. 4E), inhibited the TNF response to LPS (Fig. 4F), and was shown previously not to induce iNOS expression and to inhibit NO output in response to IFN-γ (19, 18). Although PI is an endogenous substrate for PI-phospholipase C hydrolysis leading to activation of calcium-dependent PKC, it is well established that it does not activate PKC as an exogenous agonist (28). In keeping with many previous studies, no responses were obtained by the addition of exogenous PI or lyso-PI (Fig. 4E). Thus, intact GPI is both an agonist and substrate in cell signaling, imparting two distinct signals through structurally distinct elements (PTK activation via the glycan and PKC activation via diacylglycerol). The requirement for both signals in GPI-mediated gene expression was also demonstrated by the use of specific PTK and PKC antagonists, which blocked parasite extract- and GPI-mediated TNF (Fig. 6A) and NO output (18). Furthermore, phorbol esters appeared to synergize with inositolglycan in TNF output (Fig. 6B).
How is a GPI-mediated activation signal transmitted across the membrane to the interior of the cell? GPI-anchored proteins are localized within highly specialized microdomains at the cell surface, enriched in sphingolipids and proteins such as caveolin (29–32), β-integrins (33), and Src-family PTKs, which may represent dedicated signal transducing complexes. Ligation of GPI-anchored FcγRIIIB (CD16) within these specialized domains also activates p59hck (34). These regions may contain a transmembrane receptor with a glycan-specific lectin domain that activates PTKs in response to free GPI glycans or perturbation of GPI-anchored proteins (35, 36). As GPI activates PKC and GPI-diacylglycerol is required for this, either GPI or membrane-permeant, pharmacologically active lipid hydrolysis products (e.g., phosphatidic acid or diacylglycerol) do indeed cross from the luminal to the cytoplasmic side of the plasma membrane. As malarial GPI is myristoylated on the inositol and resistant to hydrolysis by PI-phospholipase C (22, 23), a speculative possibility is that GPI is hydrolyzed at the cell surface by GPI-PLD acting as a PTK-dependent signal-activated phospholipase. Alternatively, the intact GPI may be translocated from the external surface of the cell to the interior, similar to processes during GPI biosynthesis, where a “flippase” has been postulated to account for the movement of GPI precursors from the cytoplasmic to the luminal face of the endoplasmic reticulum (37). Thus remaining issues concerning GPI-specific signaling events concern the identity of the glycan-specific receptor responsible for activation of PTKs, and determination of the mechanism by which GPI-derived fatty acids enter the signaling pathway as agonistic or antagonistic second messengers.
The differing activities of GPIs and iM4 GIPL establishes lipid-dependent signaling specificity among structurally distinct GPIs. A previous study (19), subsequently confirmed (18), showed that Leishmania GIPLs inhibited the macrophage NO response to interferon-γ and implicated the alkylacylglycerol moiety (19). Leishmanial lipophosphoglycan has also been shown to down-regulate macrophage function (38). As interferon γ induced iNOS expression is a major effector mechanism in the immunological control of Leishmania (39), it was proposed that lipophosphoglycan and GIPLs may function as mechanisms of immune evasion in this infection (19, 38). The GPI-induced PTK and PKC signals collaborate in activation of the REL-A, C-Rel, and NF-κB1 members of the NF-κB/rel family of transcription factors in vascular endothelium, macrophages, and B lymphocytes (unpublished data), leading to downstream expression of the NF-κB/rel dependent locii TNF, interleukin 1, interleukin 6, iNOS, intercellular adhesion molecule 1, and vascular adhesion molecule 1 (18, 17), implicated in malarial pathogenesis and immune dysregulation (20, 21). Malarial GPI is therefore an important pathogenicity and virulence determinant. Thus the action of structurally diverse GPI/GIPLs on macrophages may be responsible for overall features of macrophage activation and anergy in these various parasite infections.
Acknowledgments
This work received support from the United Nations Development Program/World Bank/WHO Special Programme for Research and Training in Tropical Diseases, the Ramaciotti Foundation, the W. Buckland Foundation, the Australian Academy of Science, the Deutsche Forschungsgemeinschaft, Hessisches Ministerium fur Kultur und Wissenschaft, Fonds der Chemischen Industrie, the Boehringer Ingelheim Foundation, and Siftung P.E. Kempkes. M.J.M. is a Wellcome Trust Senior Research Fellow.
ABBREVIATIONS
- iNOS
inducible NO synthase
- PDBu
phorbol 12,13-dibutyrate
- GPI
glycosylphosphatidylinositol
- GPI-PLD
GPI-specific phospholipase D
- GIPL
glycoinositolphospholipid
- PI
phosphatidylinositol
- IPG
inositolphosphoglycan
- PTK
protein tyrosine kinase
- PKC
protein kinase C
- CMW
chloroform/methanol/water
- TNF
tumor necrosis factor
- mfVSG
membrane form variant surface glycoprotein
References
- 1.Ferguson M A J, Haldar K, Cross G A M. J Biol Chem. 1985;260:4963–4968. [PubMed] [Google Scholar]
- 2.McConville M J, Ferguson M A J. Biochem J. 1993;294:305–324. doi: 10.1042/bj2940305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robinson P J, Millrain M, Antoniou J, Simpson E, Mellor A L. Nature (London) 1989;342:85–87. doi: 10.1038/342085a0. [DOI] [PubMed] [Google Scholar]
- 4.Malek T R, Fleming T J, Codias E K. Semin Immunol. 1994;6:105–113. doi: 10.1006/smim.1994.1015. [DOI] [PubMed] [Google Scholar]
- 5.Lund-Johansen F, Olweus J, Symington F W, Arli A, Thompson J S, Vilella R, Skubitz K, Horejsi V. Eur J Immunol. 1993;23:2782–2791. doi: 10.1002/eji.1830231110. [DOI] [PubMed] [Google Scholar]
- 6.Hundt M, Schmidt R E. Eur J Immunol. 1992;22:811–816. doi: 10.1002/eji.1830220327. [DOI] [PubMed] [Google Scholar]
- 7.Shenoy-Scaria A M, Kwong J, Fujita T, Olszowy M W, Shaw A S, Lublin D M. J Immunol. 1992;149:3535–3541. [PubMed] [Google Scholar]
- 8.Thomas P M, Samelson L E. J Biol Chem. 1992;267:12317–12322. [PubMed] [Google Scholar]
- 9.Garnett D, Barclay A N, Carmo A M, Beyers A D. Eur J Immunol. 1993;23:2540–2544. doi: 10.1002/eji.1830231024. [DOI] [PubMed] [Google Scholar]
- 10.Stefanova I, Corcoran M L, Horak E M, Wahl L M, Bolen J B, Horak I D. J Biol Chem. 1993;268:20725–20728. [PubMed] [Google Scholar]
- 11.Saltiel A R. J Bioenerg Biomembr. 1991;23:29–41. doi: 10.1007/BF00768837. [DOI] [PubMed] [Google Scholar]
- 12.Chan B L, Chao M V, Saltiel A R. Proc Natl Acad Sci USA. 1989;86:1756–1760. doi: 10.1073/pnas.86.6.1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Represa J, Avila M A, Miner C, Giraldez F, Romero G, Clemente R, Mato J M, Varela-Nieto I. Proc Natl Acad Sci USA. 1991;88:8016–8019. doi: 10.1073/pnas.88.18.8016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Merida I, Pratt J C, Gaulton G N. Proc Natl Acad Sci USA. 1990;87:9421–9425. doi: 10.1073/pnas.87.23.9421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schofield L, Hackett F. J Exp Med. 1993;177:145–153. doi: 10.1084/jem.177.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tachado S, Schofield L. Biochem Biohys Res Commun. 1994;205:984–991. doi: 10.1006/bbrc.1994.2763. [DOI] [PubMed] [Google Scholar]
- 17.Schofield L, Novakovic S, Gerold P, Schwarz R T, McConville M J, Tachado S D. J Immunol. 1996;156:1886–1896. [PubMed] [Google Scholar]
- 18.Tachado S D, Gerold P, McConville M J, Baldwin T, Quilici D, Schwarz R T, Schofield L. J Immunol. 1996;156:1897–1907. [PubMed] [Google Scholar]
- 19.Proudfoot L, O’Donnell C A, Liew F Y. Eur J Immunol. 1995;25:745–750. doi: 10.1002/eji.1830250318. [DOI] [PubMed] [Google Scholar]
- 20.Clark I A, Rockett K A. Parasitol Today. 1994;10:410–412. doi: 10.1016/0169-4758(94)90237-2. [DOI] [PubMed] [Google Scholar]
- 21.Berendt A R, Turner G D H, Newbold C I. Parasitol Today. 1994;10:412–414. doi: 10.1016/0169-4758(94)90238-0. [DOI] [PubMed] [Google Scholar]
- 22.Gerold P, Dieckmann-Schuppert A, Schwarz R T. J Biol Chem. 1994;269:2597–2606. [PubMed] [Google Scholar]
- 23.Gerold P, Schofield L, Blackman M, Holder A A, Schwarz R T. Mol Biochem Parasitol. 1996;75:131–143. doi: 10.1016/0166-6851(95)02518-9. [DOI] [PubMed] [Google Scholar]
- 24.McConville M J, Blackwell J M. J Biol Chem. 1991;266:15170–15179. [PubMed] [Google Scholar]
- 25.McConville M J, Collidge T A C, Ferguson M A J, Schneider P. J Biol Chem. 1993;268:15595–15604. [PubMed] [Google Scholar]
- 26.Schofield L, Vivas L, Hackett F, Gerold P, Schwarz R T, Tachado S. Ann Trop Med Parasitol. 1993;87:617–626. doi: 10.1080/00034983.1993.11812820. [DOI] [PubMed] [Google Scholar]
- 27.English B K, Ihle J N, Myracle A, Yi T. J Exp Med. 1993;178:1017–1022. doi: 10.1084/jem.178.3.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaibuchi K, Takai Y, Nishizuka Y. J Biol Chem. 1981;256:7146–7149. [PubMed] [Google Scholar]
- 29.Sargiacomo M, Sudol M, Tang Z, Lisanti M P. J Cell Biol. 1993;122:789–807. doi: 10.1083/jcb.122.4.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mayor S, Rothberg K G, Maxfield F R. Science. 1994;264:1948–1951. doi: 10.1126/science.7516582. [DOI] [PubMed] [Google Scholar]
- 31.Parpal S, Gustavsson J, StrŚlfors P. J Cell Biol. 1995;131:125–135. doi: 10.1083/jcb.131.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lisanti M P, Tang Z, Scherer P E, Kubler E, Koleske A J, Sargiacomo M. Mol Membr Biol. 1995;12:121–124. doi: 10.3109/09687689509038506. [DOI] [PubMed] [Google Scholar]
- 33.Bohuslav J, Horejsi V, Hansmann C, Stockl J, Weidle U H, Majdic O, Bartke I, Knapp W, Stockinger H. J Exp Med. 1995;181:1381–1390. doi: 10.1084/jem.181.4.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou M, Lublin D M, Link D C, Brown E J. J Biol Chem. 1995;270:13553–13560. doi: 10.1074/jbc.270.22.13553. [DOI] [PubMed] [Google Scholar]
- 35.Krauss J C, Poo H, Xue W, Mayo-Bond L, Todd R F, III, Petty H R. J Immunol. 1994;153:1769–1777. [PubMed] [Google Scholar]
- 36.Poo H, Krauss J C, Mayo-Bond L, Todd R F, III, Petty H R. J Mol Biol. 1995;247:597–603. doi: 10.1006/jmbi.1995.0166. [DOI] [PubMed] [Google Scholar]
- 37.Vidugiriene J, Menon A K. J Cell Biol. 1994;127:333–341. doi: 10.1083/jcb.127.2.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Descoteaux A, Turco S J, Sacks D L, Matlashewski G. J Immunol. 1991;146:2747–2753. [PubMed] [Google Scholar]
- 39.Stenger S, Thüring H, Röllinghoff M, Bogdan C. J Exp Med. 1994;180:783–793. doi: 10.1084/jem.180.3.783. [DOI] [PMC free article] [PubMed] [Google Scholar]