Abstract
Recruitment of circulating monocytes into the artery wall is an important feature of early atherogenesis. In vitro studies have identified a number of adhesion molecules and chemokines that may control this process but very little work has been done to evaluate their relative importance in vivo, in part because there have been no methods available of sufficient sensitivity and reliability. This paper proposes a new approach in which advantage is taken of naturally occurring or transgenically induced mutations to “mark” donor cells and to follow their fate in recipient animals using highly sensitive PCR methods. The feasibility of the approach is demonstrated by preliminary studies of monocyte recruitment into atherosclerotic lesions. However, the method should in principle be applicable to the study of any of the circulating leukocytes and their rate of entry into any tissue or tissues of interest.
The fatty streak lesion is the earliest grossly detectable lesion of human and experimental atherosclerosis and the hallmark of that lesion is the foam cell. The largest fraction of these lipid-laden cells arises from circulating monocytes that have adhered to the endothelium, penetrated into the intima, altered their phenotypic expression and begun to take up modified lipoproteins. One of the earliest responses to hypercholesterolemia in animal models is an increased adherence of circulating monocytes to the endothelium (see ref. 1 for review). The atherosclerotic lesion also contains T-lymphocytes (2) and, as at other sites of inflammation, interactions between these T-lymphocytes and macrophages and their interaction with smooth muscle cells and endothelial cells probably play a crucial role in shaping the evolution of the lesion. In vitro studies have identified a number of the factors that may control the adherence and penetration of monocytes into lesions but very little in vivo work has been done to evaluate either the rates at which these cells are recruited or the relative importance of the many adhesion molecules and chemokines that could theoretically play a role. In part this is because there have been no methods available of sufficient sensitivity and reliability to permit such measurements. Any labeling method that requires more than a minimum of manipulation of leukocytes is likely to damage or activate the cells (e.g., loading of the cells with carbon black or colloidal gold) (3). Manipulation can be avoided by injecting a tracer (e.g., tritiated thymidine) into the animal, leading to efficient labeling of stem cells in the bone marrow, followed by studies of the rates of appearance of radioactivity in circulating monocytes and in tissue macrophages. This method was most effectively exploited by van Furth et al. (4–6). However the method depends upon certain assumptions regarding the labeling of stem cells at different stages in their differentiation and the use of a rather complex multicompartmental system to derive rate constants and pool sizes. In 1963 Balner (7) irradiated C57 black mice and reconstituted their bone marrow with cells from CBA mice. He then showed that CBA-type cells (identified using specific antisera) gradually replaced C57 black peritoneal macrophages, confirming the bone marrow origin of the peritoneal macrophage. In principle this approach could be combined with PCR amplification to enhance sensitivity, but again all leukocytes would be marked and again analysis would rest on the use of complex multicompartmental analysis and assumptions as to how rapidly the bone marrow CBA cell population develops and enters the circulation.
Lewis et al. (8) labeled leukocytes in vivo by giving multiple infusions of tritiated thymidine to pigeons that were being repeatedly bled to induce rapid leukocyte formation. The harvested tritium-labeled mononuclear cells were infused into a recipient bird with atherosclerotic lesions. Autoradiography showed that two-thirds of the radiographic grains were in fact associated with foam cells, but the rest were associated with smooth muscle cells and endothelial cells. No other quantitative data were reported and no further reports have appeared; presumably the sensitivity of this approach was limited.
Thus it appears that none of the methods currently available is entirely satisfactory for quantifying monocyte recruitment into arteriosclerotic lesions or other tissues. We believe the experiments described below demonstrate the feasibility of a novel approach we are proposing, although a number of questions will undoubtedly need to be resolved before it can be reduced to practice for specific applications. The basic idea is the introduction into a recipient animal of leukocytes differing from those of the recipient by virtue of one easily identified and quantified genetic marker. The PCR is then used to quantify the donor cells, using primers that do not yield any product from the recipient’s DNA. Analysis of serial blood samples establishes the rate of disappearance of the donor cells from the bloodstream and analysis of the tissues of interest allows calculation of the rates of leukocyte entry into the tissues of interest. The remarkable sensitivity of PCR permits the detection of as few as one genetically marked cell in a million unmarked cells (9). Morgan et al. (10) exploited the sensitivity of PCR to study the persistence of tumor infiltrating lymphocytes introduced into the circulation of melanoma patients. These cells had been marked ex vivo with a neomycin resistance gene introduced using a retroviral vector, thus requiring manipulations that could alter the biological behavior of the marked cells. To minimize manipulation of cells as much as possible (to avoid activation or damage) we have elected to use a naturally occurring mutation in these preliminary experiments, transferring the cells from donor to recipient with a minimum of handling.
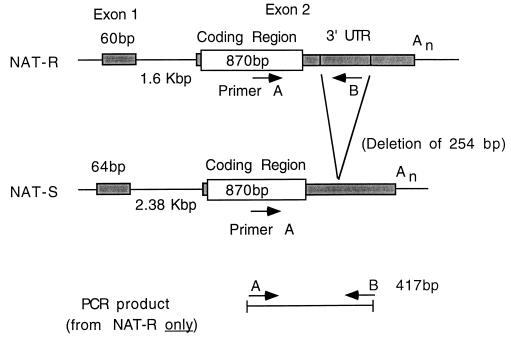
In these exploratory studies we have taken advantage of a relatively common rabbit mutation that results in total loss of the gene coding for the major form of arylamine N-acetyltransferase (NAT-R), a mutation that sharply reduces the rabbits’ ability to acetylate arylamines (11, 12). These animals do retain some residual enzyme activity but that activity is entirely attributable to a different gene, NAT-S, which is highly homologous with the wild-type gene but differs in several important respects. Most importantly, the mutant form shows a deletion of >250 bp in the 3′ untranslated region (Fig. 1). We chose one primer (primer A) near the 3′ end of the coding region and the other (primer B) within the segment deleted in the NAT-S gene so that no PCR product is generated from the mutant form.
Figure 1.
Schema showing the gene structure of the major form of rabbit arylamine-N-acetyltransferase (NAT-R) and of the minor form (NAT-S). The latter has a 254 bp deletion in the 3′ noncoding region. Consequently rabbits that only have the NAT-S form show no PCR product with the primers used in this study (see Materials and Methods).
MATERIALS AND METHODS
Experimental Animals and Preparation of Monocytes.
It has been estimated that ≈10% of New Zealand White rabbits are homozygous for a major deletion in the gene coding for the major form of NAT-R (11, 12). We screened a commercial colony of New Zealand White rabbits to select animals that were homozygous for this mutation and used them as recipients. Wild-type New Zealand White rabbits from the colony were used as donors. Monocytes were prepared from ≈150 ml of whole blood. The buffy coat was subjected to a two-step discontinuous density gradient centrifugation: Histopaque 1.077 (Sigma) followed by NycoPrep 1.068 (Accurate Chemicals). To assess purity, cells were centrifuged in Cytospin 3 (Shandon, Pittsburgh) and stained with Wright–Giemsa. The preparation was ≈70% pure, still containing some lymphocytes and a few neutrophils. The yield was ≈1 × 107 cells.
PCR.
DNA was extracted using the Easy-DNA kit (Invitrogen). Eight blood samples from a single rabbit yielded 93 ± 17 μg DNA per ml (mean ± SD). Reproducibility of DNA extraction from aorta was evaluated from results on eight successive thoracic segments of approximately equal area (net weight 33 ± 5.6 mg); DNA content was 0.74 ± 0.15 μg/mg (mean ± SD).
The primers used are shown in Fig. 1. Primer A, the sense primer, corresponds to nucleotides 833–857, a segment of the coding sequence that is identical in the NAT-R and NAT-S genes (11, 12). Primer B, the antisense primer, corresponds to nucleotides 1227–1249 of NAT-R; these nucleotides are deleted in the NAT-S gene and thus the latter yields no PCR product. These primers amplify a 417-bp sequence. Amplification reactions were carried out in a 50 μl volume containing 10 mM Tris (pH 8.3), 50 mM KCl, 1.5 mM MgCl2, 200 μM each of the dNTPs, 5 pmol each of the two primers, 1.25 units of Taq DNA polymerase, and 0.1–1.0 μg genomic DNA. Reactions were performed in an Ericomp thermal cycler with heated lid. The first denaturing cycle was 95°C for 3 min, followed by 30 cycles of annealing at 60°C for 1 min, extension at 72°C for 1 min, and denaturation at 94°C for 1 min. Cycling was concluded at 72°C for 10 min. For electrophoresis, one-third of the reaction mixture was mixed with bromphenol blue and loaded onto a 1.5% agarose gel.
For quantitative detection of PCR products, Southern blots were exposed to UV radiation and hybridized with a solution containing 2 × 107 cpm/ml of a 32P-labeled internal probe. This probe, corresponding to nucleotides 1075–1092 (a part of the sequence deleted in the NAT-S form), was end-labeled with γ-32P-ATP and T4 polynucleotide kinase to yield a specific activity of ≈2 × 109 cpm/μg. After overnight hybridization, the blot was washed extensively with large volumes of decreasing concentrations of standard saline citrate (0.15 M sodium chloride/0.015 M sodium citrate) containing 0.1% SDS. Autoradiograms were made at −80°C for 20 min to 24 hr. For quantification, the individual bands were cut out and assayed in a scintillation counter.
Competitive PCR and Calculation of Numbers of Donor Leukocytes in Lesions.
The internal standard used was amplified by the same primers used to amplify the NAT-R gene, but, by virtue of a 250-bp segment of foreign DNA inserted into the SmaI site, yielded a 667-bp product easily separated from the NAT-R amplification product of 417 bp. Quantification was achieved by titrating a constant amount of the unknown target sequence against successive dilutions of the internal standard. The amount of internal standard yielding equivalent product to that obtained from the unknown sample defines the amount of target in μmoles per sample. Assuming two copies per cell there will be 3.3 × 10−18 μmoles of NAT-R (or any other allele) per donor cell. Thus μmoles NAT-R per sample divided by 3.3 × 10−18 μmol/cell yields number of NAT-R positive cells per sample (A). Total DNA in the sample analyzed divided by DNA per cell (6 × 10−6 μg/cell) yields total number of cells per sample (B). Then A/B × 100 yields the percent donor cells in recipient test sample or A/B × 106 yields donor cells per million cells in recipient test sample.
RESULTS AND DISCUSSION
Sensitivity and Reproducibility of PCR.
To test the sensitivity of our method, PCR was carried out on progressive dilutions of genomic DNA purified from the blood of a wild-type rabbit. The amounts used ranged from 50 ng–1 pg. The 32P-probe readily detected PCR product down to 10 pg and there was a good linear relationship over a range of values between 0.1 and 10 ng (data not shown).
We also tested the sensitivity of the method by diluting mononuclear cells from a wild-type rabbit with mononuclear cells from a mutant rabbit, the ratios ranging from 1:500–1:1,000,000. The radioactive probe for the 417-bp product was able to clearly detect a band at a dilution of five cells in one million unmarked cells (data not shown).
Disappearance of Donor Cells from the Bloodstream of Recipient Animals.
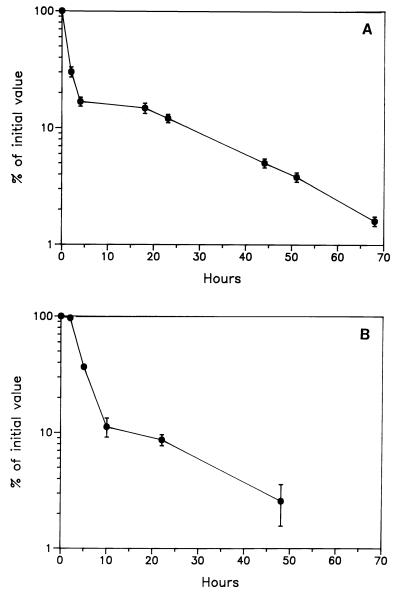
Monocytes prepared from an NAT-R rabbit by discontinuous density gradient centrifugation, as described in Materials and Methods, were resuspended in 2 ml of sterile PBS and injected intravenously into a mutant (NAT-S negative) rabbit. Recipient blood samples were drawn at time intervals, DNA was extracted from 0.25 ml of blood and 1 μg was taken for PCR. The sample drawn prior to monocyte infusion gave values not differing from background (≈50 cpm). As shown in Fig. 2A, there was a rapid initial fall in circulating donor cells, followed by a slow late phase of disappearance. The agreement between duplicates in Fig. 2A was excellent. More than 75% of the administered cells were “lost” over the first 2 hr in agreement with the results of others and presumably reflecting mixing with the large marginated pool of leukocytes (13, 14). The slower subsequent decay corresponded to a half-life of ≈24 hr. These findings are consistent with those of Ohgami et al. (13) and of Doherty et al. (14) who reported the half-life of indium-labeled heterologous monocytes to be 24 and 30 hr, respectively.
Figure 2.
(A) Time course of the disappearance of donor monocytes, purified from the blood of a wild-type donor (NAT-R) and injected intravenously into a mutant recipient (NAT-S). Monocytes were purified from ≈150 ml of donor blood and ≈1 × 107 cells were injected. Results are expressed as a percentage of the value at 30 min. (B) Time course of the disappearance of donor monocytes from the blood of a mutant recipient rabbit (NAT-S) after intravenous injection of 45 ml of whole blood from a wild-type donor (NAT-R). Monocytes were recovered from timed blood samples by first isolating the mononuclear cell fraction by density gradient centrifugation and then allowing the monocytes to adhere to plastic plates previously coated with microexudate secreted by BHK cells. Results are expressed relative to the value at 23 min. Values for duplicate analyses at each time point are shown.
A major concern in experiments of this kind is the possibility of damage to or activation of leukocytes during their preparation. In an effort to assess the extent of damage or activation of monocytes during purification we carried out an experiment that should minimize any damage or activation. This experiment was done by transfusing whole blood (45 ml) from an NAT-R donor into a mutant recipient and then taking timed blood samples from which monocytes were purified prior to PCR. In this way there was virtually no handling of the leukocytes prior to their transfusion into the recipient; purification of the monocytes from each timed sample could then be carried out without the need to take precautions to prevent activation. We prepared the mononuclear cells using discontinuous density gradient techniques and then allowed the monocytes to adhere to a plastic surface. DNA was prepared from these adherent monocytes and subjected to PCR. In this way we were able to generate a curve for the disappearance of monocytes that had been subjected to the least possible manipulation. As shown in Fig. 2B the general shape of the disappearance curve was very similar to that obtained when monocytes were purified from the donor prior to injection (compare Fig. 2A). The late phase of disappearance showed that monocytes had about the same half-life in both cases, i.e., ≈24 hr. The rate of the initial fall, which is presumed to represent mixing with the marginated pool of leukocytes, was comparable in degree but the rate was somewhat higher for the purified monocytes. Obviously more work will be needed to determine whether there is a systematic difference between purified monocytes and monocytes that have not been manipulated. If there is, other methods for isolation of the monocytes will need to be used, e.g., use of magnetic beads coated with monoclonal antibodies against lymphocytes and granulocytes to purify monocytes by negative selection. We would propose that for each application an experiment like that shown in Fig. 2B always be used as a “gold standard” against which to compare the behavior of leukocytes that have been purified (by whatever method) prior to transfusion into the recipients.
These studies were done using allogeneic animals and consequently the recipients will begin to mount an immune response after the introduction of foreign cells. However, it is unlikely that the immune response would importantly affect the results during the first 24 hr because the number of recipient cells recognizing the foreign antigens should be very small initially. Two points supporting this interpretation are: (i) That after the rapid initial mixing phase there was no obvious increase in the slope of the slow phase as might be expected as the immune response is mounted; (ii) That Issekutz et al. (15) used autologous monocytes and found a half-life of 34.1 hr and Doherty et al. (14), using heterologous monocytes, found a similar half-life (30 hr). Future studies should be done whenever possible in congenic animals differing only with respect to the marker gene employed so that protocols can extend over days or weeks. For example, one could adapt the method for use in mice, by using C57BL/6 donors and recipients lacking the low density lipoprotein receptor that have been back-crossed into a C57BL/6 genetic background. An even better approach would be to exploit the fact that the mouse sex-determining gene (sry), cloned by Gubbay et al. (16), is uniquely found on the Y chromosome. This clearly makes it the marker of choice for studies in the mouse (or in any species in which the sex-determining gene has been sequenced) because cells of male donors can be injected into female recipients that are totally congenic (except for sry and other sex-specific genes but these are probably not expressed in the adult). This approach has been successfully applied to follow the fate of transfused leukocytes (17). Thus, there is virtually no concern with respect to immune responses.
Tissue Uptake of Infused Monocytes.
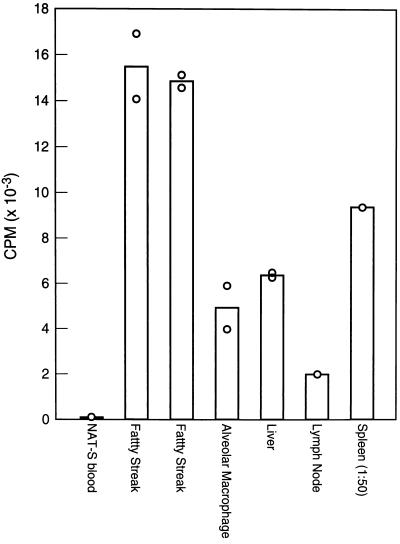
To determine whether the method was sufficiently sensitive to detect the uptake of infused monocytes into tissues and, more specifically, into atherosclerotic lesions, NAT-R monocytes were introduced into an NAT-S rabbit that had been on a 1% cholesterol diet for 18 wk. Donor monocytes were prepared by discontinuous density gradient centrifugation and injected via an ear vein. The recipient was sacrificed at 65 hr with a bolus injection of Nembutal. Alveolar macrophages were harvested by pulmonary lavage and the animal was perfused with 3 liters of PBS containing 2 mM EDTA. Samples of tissues (liver, spleen, alveolar macrophages, lymph nodes, and atherosclerotic lesions) were collected and intimal lesions were stripped from the aortic arch and from the thoracic aorta. DNA was extracted from 20–30 mg of each sample and l μg of DNA was subjected to PCR (except in the case of spleen where only 20 ng was needed because of the large number of donor cells present). As shown in Fig. 3, the presence of donor monocytes was readily demonstrated in all of the tissues examined including atherosclerotic lesions. As a control, we used a sample of blood taken from the NAT-S recipient just prior to the infusion of monocytes and this was clearly negative. Since the background radioactivity under our conditions was only ≈50 cpm, while the values for the two pooled aortic lesion samples was ≈15,000 cpm, we should be able to measure donor cell uptake in samples even two orders of magnitude smaller or at much earlier stages of lesion development. Note that the duplicate values agreed quite closely.
Figure 3.
Recovery of donor cells in recipient tissues 65 hr after injection of purified NAT-R positive monocytes into an NAT-S rabbit. The relative quantities of PCR products are expressed as cpm per μg of DNA from each tissue (except in the case of spleen, where the data represent cpm per 20 ng of DNA). Each open circle represents a value for a sample of DNA carried separately through PCR and hybridization.
Estimating the Absolute Number of Leukocytes Taken up into Lesions.
Two additional studies were done in which whole blood was given by transfusion and in which competitive PCR was used to quantify leukocyte entry into atherosclerotic lesions. Obviously this measures uptake of all leukocytes, not just monocytes, but it provides an estimate of the overall sensitivity and reproducibility of this approach using unmanipulated leukocytes. Actually, at this early stage, fatty streak lesions contain predominantly monocytes, monocyte-derived foam cells, and a few T-lymphocytes but no B-lymphocytes nor granulocytes. Consequently the values should closely approximate monocyte entry. In these studies, instead of stripping the intimal lesions, we took full thickness samples of aorta.
The first recipient animal had been on a 1% cholesterol diet for only 10 wk and showed only minimal lesions in the thoracic aorta. The abdominal aorta and the aortic arch showed no grossly detectable lesions. Only one pair of intercostal artery ostia stained positively with Sudan IV. We dissected equal sized segments surrounding one of those ostia and the ostia above and below it. As shown in Fig. 4, a positive PCR signal was obtained only from the segment that showed Sudan IV staining (lane 6). Radioassay of this band showed 837 cpm; the bands from lanes 5 and 7 showed no detectable radioactivity above background. Using competitive PCR, we calculated that 89 per million cells in this early fatty streak were donor leukocytes. Since the blood introduced initially was diluted ≈1:7 by recipient cells, the total number of newly recruited leukocytes in this lesion would be ≈623 per million.
Figure 4.
Autoradiogram showing the specific 417-bp PCR product from aortic segments and from the spleen of a mutant rabbit that received an infusion of 40 ml of whole blood from an NAT-R donor via the ear vein 70 hr previously. One μg of DNA was used for PCR. Lanes 1 and 2: abdominal aorta; lanes 3–7: thoracic aorta; lane 8: aortic arch (which was free of lesions); lanes 9 and 10: two different preparations of DNA from spleen; lanes 11 and 12: positive controls (0.1 μg of DNA isolated from the donor’s blood).
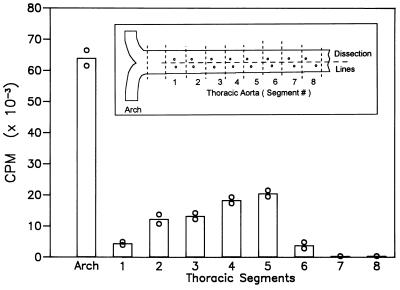
We did a similar study in a rabbit fed 1% cholesterol for 18 wk to measure leukocyte infiltration into more advanced lesions. This animal showed very extensive and prominent lesions in the aortic arch as well as lesions at most of the intercostal artery ostia. As shown in Fig. 5, the aortic arch contained many more donor leukocytes than the artery segments surrounding the intercostal ostia. Note the close agreement between the values for duplicate analyses; two different DNA samples, separately amplified and hybridized, were used for these two analyses. Aliquots of extracted DNA were used for competitive PCR to estimate the absolute number of leukocytes in the lesions, as described in Materials and Methods. That analysis showed that in the aortic arch there were 11,200 donor leukocytes per 1 × 106 aortic cells (full-thickness samples). Thoracic aortic segment #5, the most strongly positive segment by PCR, contained 3,860 donor leukocytes per 1 × 106 cells. Stating these results differently, newly entered leukocytes accounted for >1% of all the cells in the aortic arch and up to 0.4% of all cells in the most advanced of the thoracic lesions.
Figure 5.
Leukocyte uptake into the aortic lesions of a mutant cholesterol-fed rabbit (18 wk) 70 hr after transfusion of 45 ml of whole blood from a wild-type donor. Data represent the amount of 32P-labeled probe hybridizing with PCR products generated from 1 μg of aortic DNA. ○, Values for duplicate DNA samples carried separately through PCR and hybridization. As indicated in the inset, the successive thoracic segments represented equal-length samples along the aorta.
At first glance these values seem high but it must be kept in mind that these lesions are not at steady state. Exact values are not available but we estimate that on a 1% cholesterol diet the fatty streak lesions at least double in surface area between weeks 12 and 18 (and of course more than double in volume). If the expansion were proportional to the volume of the lesion and it doubled in 6 wk, that would correspond to an increase of 1.6% per day. Thus the rate of lesion expansion is compatible with continuing recruitment and retention of leukocytes at the rate calculated. However, the rate of increase in lesion size and volume will vary over time, and in more advanced lesions some of the increase will reflect smooth muscle cell replication and matrix deposition. Time course studies of lesion growth and rates of monocyte recruitment will be needed to establish how much turnover of monocyte/macrophages (death or egress from the lesion) occurs.
Further Applications in the Study of Atherogenesis.
What are some examples of the biological questions that might be answered using this new approach? While the recruitment of monocytes is obviously an essential component of fatty streak generation, we have almost no quantitative measurements of the rate at which monocytes are recruited in vivo. Does monocyte recruitment accelerate as the lesions mature? Does recruitment stop after a certain point or is there continuing recruitment of new monocytes into even well established lesions? In vitro studies have already implicated certain adhesion molecules, such as VCAM-1 (18, 19), in the recruitment of monocytes but we do not have data evaluating the relative importance of various selectins and integrins under in vivo conditions, where flow conditions are quite different and where the other formed elements may influence adherence and penetration. One way to evaluate these would be to administer blocking antibodies just prior to and during the early disappearance of donor monocytes and to compare the results with those in control animals given preimmune serum or isotype-matched immunoglobulin controls. Another example: In vitro studies show that oxidized low density lipoprotein can itself act as a chemoattractant (20) or stimulate the release of a chemoattractant (MCP-1) from endothelial cells (21). Is the effectiveness of antioxidants in animal models due in part to inhibition of these effects in vivo? Comparison of monocyte recruitment in control animals with that in antioxidant-treated animals should provide an answer.
When the method is applied in congenic animals, where immune reaction is not a problem and the experiments can last for longer time periods, it should be possible to answer questions about the persistence of newly recruited monocytes in lesions. As shown above, >99% of the administered monocytes have exited the vascular system in a few days. Measurements of newly recruited cells in lesions after 1, 2, or 3 days should provide a baseline against which to measure the numbers found in other animals allowed to go on for additional days (or weeks) to see how long these newly recruited cells persist.
How long after recruitment do monocytes become loaded with fat droplets and acquire the properties of foam cells? In situ hybridization using an antisense RNA probe that will bind only to the wild-type gene might make it possible to answer this question.
Application to Other Biological Problems.
In principle this approach should be applicable to the study of any of the leukocytes, provided acceptable methods for isolating them and transfusing them can be developed. The kinetics of entry into any organ or tissue space should be measurable. Thus, studies of recruitment of different classes of leukocytes into sites of inflammation (joints, alveoli, peritoneal cavity, etc.) should be possible.
The origin of the microglia in the brain has been extensively investigated, using a broad array of methods but the issue remains controversial: To what extent do they derive from monocytes and when? (see ref. 22 for review). The least ambiguous approach was that of de Groot et al. (23), who reconstituted the bone marrow from a transgenic mouse expressing bacteriophage λ. However this did not distinguish among the several leukocyte lineages. The method proposed here might allow specific measurement of the rate of replacement by circulating monocytes (or lymphocytes) at different ages and in the absence or presence of inflammation. There are undoubtedly many other potential applications but each will predictably pose special problems in reduction to practice.
Acknowledgments
We thank Dr. Myron I. Cybulsky of the University of Toronto for his valuable comments and suggestions and Ms. Jennifer Pattison for her expert technical assistance. This work was supported in part by the National Heart, Lung, and Blood Institute (Grant HL-14197) and in part by the Sam and Rose Stein Institute for Research on Aging.
ABBREVIATIONS
- NAT
arylamine N-acetyltransferase
- NAT-R
the major form of NAT in the rabbit
- NAT-S
a common polymorphic form of NAT showing a deletion of >250 bp in the 3′ untranslated region
References
- 1.Steinberg D. In: Atherosclerosis Reviews. Stokes J III, Mancini M, editors. Vol. 18. New York: Raven; 1988. pp. 1–23. [Google Scholar]
- 2.Jonasson L, Holm J, Skalli O, Bondjers G, Hansson G K. Arteriosclerosis (Dallas) 1986;6:131–138. doi: 10.1161/01.atv.6.2.131. [DOI] [PubMed] [Google Scholar]
- 3.Roser B. J Reticuloendothel Soc. 1970;8:139–161. [PubMed] [Google Scholar]
- 4.van Furth R, Cohn Z A. J Exp Med. 1968;128:415–433. doi: 10.1084/jem.128.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Furth R, Diesselhoff-den Dulk M C, Raeburn J A, van Zwet T L, Crofton R, van Oud Alblas A B. In: Mononuclear Phagocytes: Functional Aspects. van Furth R, editor. Boston: Kluwer; 1980. pp. 279–298. [Google Scholar]
- 6.van Furth R, Diesselhoff-den Dulk M M C, Sluter W, van Diesel J T. In: Mononuclear Phagocytes. van Furth R, editor. Hingham, MA: Kluwer; 1985. pp. 201–208. [Google Scholar]
- 7.Balner H. Transplantation. 1963;1:217–223. doi: 10.1097/00007890-196301020-00009. [DOI] [PubMed] [Google Scholar]
- 8.Lewis J C, Taylor R G, Jerome W G. Ann NY Acad Sci. 1985;454:91–100. doi: 10.1111/j.1749-6632.1985.tb11847.x. [DOI] [PubMed] [Google Scholar]
- 9.Crescenzi M, Seto M, Herzig G P, Weiss P D, Griffith R C, Korsmeyer S J. Proc Natl Acad Sci USA. 1988;85:4869–4873. doi: 10.1073/pnas.85.13.4869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morgan R A, Cornetta K, Anderson W F. Hum Gene Ther. 1990;1:135–149. doi: 10.1089/hum.1990.1.2-135. [DOI] [PubMed] [Google Scholar]
- 11.Sasaki Y, Ohsako S, Deguchi T. J Biol Chem. 1991;266:13243–13250. [PubMed] [Google Scholar]
- 12.Blum M, Grant D M, Demierre A, Meyer U A. Proc Natl Acad Sci USA. 1989;86:9554–9557. doi: 10.1073/pnas.86.23.9554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ohgami M, Doerschuk C M, Gie R P, English D, Hogg J C. J Appl Physiol. 1991;70:152–157. doi: 10.1152/jappl.1991.70.1.152. [DOI] [PubMed] [Google Scholar]
- 14.Doherty D E, Downey G P, Worthen G S, Haslett C, Henson P M. Lab Invest. 1988;59:200–213. [PubMed] [Google Scholar]
- 15.Issekutz T B, Issekutz A C, Movat H Z. Am J Pathol. 1981;103:47–55. [PMC free article] [PubMed] [Google Scholar]
- 16.Gubbay J, Collignon J, Koopman P, Capel B, Economan A, Munsterberg A, Vivian N, Goodfellow P, Lovell-Badge R. Nature (London) 1990;346:245–250. doi: 10.1038/346245a0. [DOI] [PubMed] [Google Scholar]
- 17.Goodarzi M O, Lee T H, Pallavicini M G, Donegan E A, Busch M P. Transfusion. 1995;35:145–149. doi: 10.1046/j.1537-2995.1995.35295125737.x. [DOI] [PubMed] [Google Scholar]
- 18.Cybulsky M I, Gimbrone M A., Jr Science. 1991;251:788–791. doi: 10.1126/science.1990440. [DOI] [PubMed] [Google Scholar]
- 19.Li H, Cybulsky M I, Gimbrone M A, Jr, Libby P. Arterioscler Thromb. 1993;13:197–204. doi: 10.1161/01.atv.13.2.197. [DOI] [PubMed] [Google Scholar]
- 20.Quinn M T, Parthasarathy S, Fong L G, Steinberg D. Proc Natl Acad Sci USA. 1987;84:2995–2998. doi: 10.1073/pnas.84.9.2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cushing S D, Berliner J A, Valente A J, Territo M C, Navab M, Parhami F, Gerrity R, Schwartz C J, Fogelman A M. Proc Natl Acad Sci USA. 1990;87:5134–5138. doi: 10.1073/pnas.87.13.5134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Federoff S. In: Neuroglia. Kettenmann H, Ransom B R, editors. New York: Oxford Univ. Press; 1995. pp. 162–181. [Google Scholar]
- 23.de Groot C J A, Huppes W, Sminia T, Kraal G, Dijkstra C D. Glia. 1992;6:301–309. doi: 10.1002/glia.440060408. [DOI] [PubMed] [Google Scholar]