Abstract
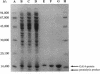
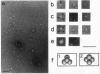
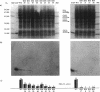
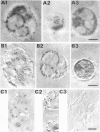
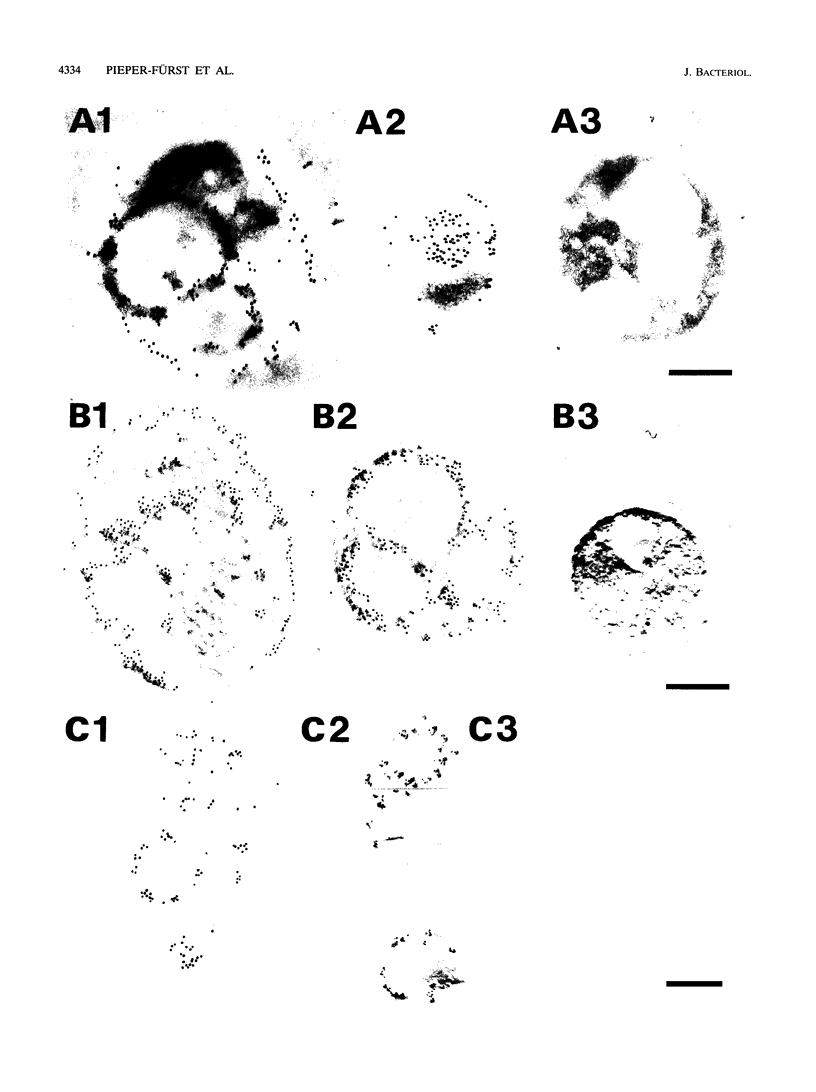
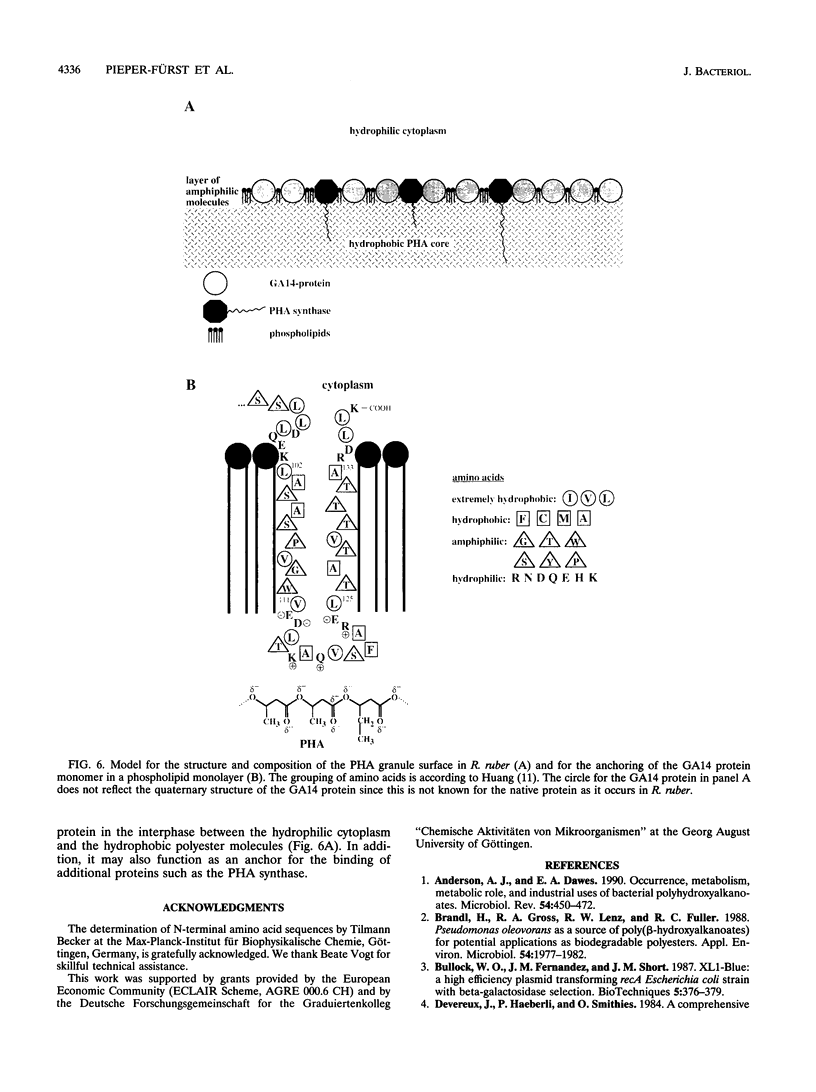
The N-terminal amino acid sequence of the polyhydroxyalkanoic acid (PHA) granule-associated M(r)-15,500 protein of Rhodococcus ruber (the GA14 protein) was analyzed. The sequence revealed that the corresponding structural gene is represented by open reading frame 3, encoding a protein with a calculated M(r) of 14,175 which was recently localized downstream of the PHA synthase gene (U. Pieper and A. Steinbüchel, FEMS Microbiol. Lett. 96:73-80, 1992). A recombinant strain of Escherichia coli XL1-Blue carrying the hybrid plasmid (pSKXA10*) with open reading frame 3 overexpressed the GA14 protein. The GA14 protein was subsequently purified in a three-step procedure including chromatography on DEAE-Sephacel, phenyl-Sepharose CL-4B, and Superose 12. Determination of the molecular weight by gel filtration as well as electron microscopic studies indicates that a tetrameric structure of the recombinant, native GA14 protein is most likely. Immunoelectron microscopy demonstrated a localization of the GA14 protein at the periphery of PHA granules as well as close to the cell membrane in R. ruber. Investigations of PHA-leaky and PHA-negative mutants of R. ruber indicated that expression of the GA14 protein depended strongly on PHA synthesis.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson A. J., Dawes E. A. Occurrence, metabolism, metabolic role, and industrial uses of bacterial polyhydroxyalkanoates. Microbiol Rev. 1990 Dec;54(4):450–472. doi: 10.1128/mr.54.4.450-472.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandl H., Gross R. A., Lenz R. W., Fuller R. C. Pseudomonas oleovorans as a Source of Poly(beta-Hydroxyalkanoates) for Potential Applications as Biodegradable Polyesters. Appl Environ Microbiol. 1988 Aug;54(8):1977–1982. doi: 10.1128/aem.54.8.1977-1982.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duhamel R. C., Johnson D. A. Use of nonfat dry milk to block nonspecific nuclear and membrane staining by avidin conjugates. J Histochem Cytochem. 1985 Jul;33(7):711–714. doi: 10.1177/33.7.2409130. [DOI] [PubMed] [Google Scholar]
- Ellar D., Lundgren D. G., Okamura K., Marchessault R. H. Morphology of poly-beta-hydroxybutyrate granules. J Mol Biol. 1968 Aug 14;35(3):489–502. doi: 10.1016/s0022-2836(68)80009-9. [DOI] [PubMed] [Google Scholar]
- Gerngross T. U., Reilly P., Stubbe J., Sinskey A. J., Peoples O. P. Immunocytochemical analysis of poly-beta-hydroxybutyrate (PHB) synthase in Alcaligenes eutrophus H16: localization of the synthase enzyme at the surface of PHB granules. J Bacteriol. 1993 Aug;175(16):5289–5293. doi: 10.1128/jb.175.16.5289-5293.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griebel R., Smith Z., Merrick J. M. Metabolism of poly-beta-hydroxybutyrate. I. Purification, composition, and properties of native poly-beta-hydroxybutyrate granules from Bacillus megaterium. Biochemistry. 1968 Oct;7(10):3676–3681. doi: 10.1021/bi00850a047. [DOI] [PubMed] [Google Scholar]
- Haywood G. W., Anderson A. J., Williams D. R., Dawes E. A., Ewing D. F. Accumulation of a poly(hydroxyalkanoate) copolymer containing primarily 3-hydroxyvalerate from simple carbohydrate substrates by Rhodococcus sp. NCIMB 40126. Int J Biol Macromol. 1991 Apr;13(2):83–88. doi: 10.1016/0141-8130(91)90053-w. [DOI] [PubMed] [Google Scholar]
- KAUDEWITZ F. [The inactivating and mutagenic effect of nitrous acid on cells of Escherichia coli]. Z Naturforsch B. 1959 Aug-Sep;14B:528–537. [PubMed] [Google Scholar]
- Konigsberg W. H., Henderson L. Removal of sodium dodecyl sulfate from proteins by ion-pair extraction. Methods Enzymol. 1983;91:254–259. doi: 10.1016/s0076-6879(83)91022-4. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- LUNDGREN D. G., PFISTER R. M., MERRICK J. M. STRUCTURE OF POLY-BETA-HYDROXYBUTYRIC ACID GRANULES. J Gen Microbiol. 1964 Mar;34:441–446. doi: 10.1099/00221287-34-3-441. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Liebergesell M., Schmidt B., Steinbüchel A. Isolation and identification of granule-associated proteins relevant for poly(3-hydroxyalkanoic acid) biosynthesis in Chromatium vinosum D. FEMS Microbiol Lett. 1992 Dec 1;78(2-3):227–232. doi: 10.1016/0378-1097(92)90031-i. [DOI] [PubMed] [Google Scholar]
- Mayer F., Wallace J. C., Keech D. B. Further electron microscope studies on pyruvate carboxylase. Eur J Biochem. 1980 Nov;112(2):265–272. doi: 10.1111/j.1432-1033.1980.tb07202.x. [DOI] [PubMed] [Google Scholar]
- Olmsted J. B. Affinity purification of antibodies from diazotized paper blots of heterogeneous protein samples. J Biol Chem. 1981 Dec 10;256(23):11955–11957. [PubMed] [Google Scholar]
- Pieper U., Steinbüchel A. Identification, cloning and sequence analysis of the poly(3-hydroxyalkanoic acid) synthase gene of the gram-positive bacterium Rhodococcus ruber. FEMS Microbiol Lett. 1992 Sep 1;75(1):73–79. doi: 10.1016/0378-1097(92)90459-2. [DOI] [PubMed] [Google Scholar]
- Roth J., Bendayan M., Carlemalm E., Villiger W., Garavito M. Enhancement of structural preservation and immunocytochemical staining in low temperature embedded pancreatic tissue. J Histochem Cytochem. 1981 May;29(5):663–671. doi: 10.1177/29.5.6166664. [DOI] [PubMed] [Google Scholar]
- SCHLEGEL H. G., KALTWASSER H., GOTTSCHALK G. [A submersion method for culture of hydrogen-oxidizing bacteria: growth physiological studies]. Arch Mikrobiol. 1961;38:209–222. [PubMed] [Google Scholar]
- Schlegel H. G., Lafferty R., Krauss I. The isolation of mutants not accumulating poly-beta-hydroxybutyric acid. Arch Mikrobiol. 1970;71(3):283–294. doi: 10.1007/BF00410161. [DOI] [PubMed] [Google Scholar]
- Steinbüchel A., Hustede E., Liebergesell M., Pieper U., Timm A., Valentin H. Molecular basis for biosynthesis and accumulation of polyhydroxyalkanoic acids in bacteria. FEMS Microbiol Rev. 1992 Dec;9(2-4):217–230. doi: 10.1111/j.1574-6968.1992.tb05841.x. [DOI] [PubMed] [Google Scholar]
- Steinbüchel A., Schlegel H. G. Physiology and molecular genetics of poly(beta-hydroxy-alkanoic acid) synthesis in Alcaligenes eutrophus. Mol Microbiol. 1991 Mar;5(3):535–542. doi: 10.1111/j.1365-2958.1991.tb00725.x. [DOI] [PubMed] [Google Scholar]
- Timm A., Steinbüchel A. Formation of polyesters consisting of medium-chain-length 3-hydroxyalkanoic acids from gluconate by Pseudomonas aeruginosa and other fluorescent pseudomonads. Appl Environ Microbiol. 1990 Nov;56(11):3360–3367. doi: 10.1128/aem.56.11.3360-3367.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentine R. C., Shapiro B. M., Stadtman E. R. Regulation of glutamine synthetase. XII. Electron microscopy of the enzyme from Escherichia coli. Biochemistry. 1968 Jun;7(6):2143–2152. doi: 10.1021/bi00846a017. [DOI] [PubMed] [Google Scholar]
- Vogelstein B., Gillespie D. Preparative and analytical purification of DNA from agarose. Proc Natl Acad Sci U S A. 1979 Feb;76(2):615–619. doi: 10.1073/pnas.76.2.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]