Abstract
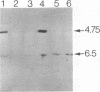
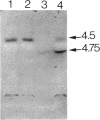
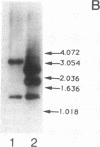
The gene encoding the serine cycle hydroxypyruvate reductase of Methylobacterium extorquens AM1 was isolated by using a synthetic oligonucleotide with a sequence based on a known N-terminal amino acid sequence. The cloned gene was inactivated by insertion of a kanamycin resistance gene, and recombination of this insertion derivative with the wild-type gene produced a serine cycle hydroxypyruvate reductase null mutant. This mutant had lost its ability to grow on C-1 compounds but retained the ability to grow on C-2 compounds, showing that the hydroxypyruvate reductase operating in the serine cycle is not involved in the conversion of acetyl coenzyme A to glycine as previously proposed. A second hydroxypyruvate-reducing enzyme with a low level of activity was found in M. extorquens AM1; this enzyme was able to interconvert glyoxylate and glycollate. The gene encoding hydroxypyruvate reductase was shown to be located about 3 kb upstream of two other serine cycles genes encoding phosphoenolpyruvate carboxylase and malyl coenzyme A lyase.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Chistoserdov A. Y., Tsygankov Y. D., Lidstrom M. E. Genetic organization of methylamine utilization genes from Methylobacterium extorquens AM1. J Bacteriol. 1991 Sep;173(18):5901–5908. doi: 10.1128/jb.173.18.5901-5908.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chistoserdova L. V., Lidstrom M. E. Purification and characterization of hydroxypyruvate reductase from the facultative methylotroph Methylobacterium extorquens AM1. J Bacteriol. 1991 Nov;173(22):7228–7232. doi: 10.1128/jb.173.22.7228-7232.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditta G., Schmidhauser T., Yakobson E., Lu P., Liang X. W., Finlay D. R., Guiney D., Helinski D. R. Plasmids related to the broad host range vector, pRK290, useful for gene cloning and for monitoring gene expression. Plasmid. 1985 Mar;13(2):149–153. doi: 10.1016/0147-619x(85)90068-x. [DOI] [PubMed] [Google Scholar]
- Dunstan P. M., Anthony C., Drabble W. T. Microbial metabolism of C 1 and C 2 compounds. The involvement of glycollate in the metabolism of ethanol and of acetate by Pseudomonas AM1. Biochem J. 1972 Jun;128(1):99–106. doi: 10.1042/bj1280099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunstan P. M., Anthony C., Drabble W. T. Microbial metabolism of C 1 and C 2 compounds. The role of glyoxylate, glycollate and acetate in the growth of Pseudomonas AM1 on ethanol and on C 1 compounds. Biochem J. 1972 Jun;128(1):107–115. doi: 10.1042/bj1280107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunstan P. M., Anthony C. Microbial metabolism of C1 and C2 compounds. The role of acetate during growth of Pseudomonas AM1 on C1 compounds, ethanol and beta-hydroxybutyrate. Biochem J. 1973 Apr;132(4):797–801. doi: 10.1042/bj1320797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulton G. L., Nunn D. N., Lidstrom M. E. Molecular cloning of a malyl coenzyme A lyase gene from Pseudomonas sp. strain AM1, a facultative methylotroph. J Bacteriol. 1984 Nov;160(2):718–723. doi: 10.1128/jb.160.2.718-723.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harder W., Quayle J. R. The biosynthesis of serine and glycine in Pseudomonas AM1 with special reference to growth on carbon sources other than C1 compounds. Biochem J. 1971 Mar;121(5):753–762. doi: 10.1042/bj1210753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heptinstall J., Quayle J. R. Pathways leading to and from serine during growth of Pseudomonas AM1 on C1 compounds or succinate. Biochem J. 1970 Apr;117(3):563–572. doi: 10.1042/bj1170563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KORNBERG H. L. Selective utilization of metabolic routes by Escherichia coli. Cold Spring Harb Symp Quant Biol. 1961;26:257–260. doi: 10.1101/sqb.1961.026.01.032. [DOI] [PubMed] [Google Scholar]
- Kalb V. F., Jr, Bernlohr R. W. A new spectrophotometric assay for protein in cell extracts. Anal Biochem. 1977 Oct;82(2):362–371. doi: 10.1016/0003-2697(77)90173-7. [DOI] [PubMed] [Google Scholar]
- Knauf V. C., Nester E. W. Wide host range cloning vectors: a cosmid clone bank of an Agrobacterium Ti plasmid. Plasmid. 1982 Jul;8(1):45–54. doi: 10.1016/0147-619x(82)90040-3. [DOI] [PubMed] [Google Scholar]
- Large P. J., Quayle J. R. Microbial growth on C(1) compounds. 5. Enzyme activities in extracts of Pseudomonas AM1. Biochem J. 1963 May;87(2):386–396. doi: 10.1042/bj0870386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lidstrom M. E., Tsygankov Y. D. Molecular genetics of methylotrophic bacteria. Biotechnology. 1991;18:273–304. doi: 10.1016/b978-0-7506-9188-8.50019-x. [DOI] [PubMed] [Google Scholar]
- McNerney T., O'connor M. L. Regulation of enzymes associated with C-1 metabolism in three facultative methylotrophs. Appl Environ Microbiol. 1980 Aug;40(2):370–375. doi: 10.1128/aem.40.2.370-375.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinkoth J., Wahl G. Hybridization of nucleic acids immobilized on solid supports. Anal Biochem. 1984 May 1;138(2):267–284. doi: 10.1016/0003-2697(84)90808-x. [DOI] [PubMed] [Google Scholar]
- Nunn D. N., Lidstrom M. E. Isolation and complementation analysis of 10 methanol oxidation mutant classes and identification of the methanol dehydrogenase structural gene of Methylobacterium sp. strain AM1. J Bacteriol. 1986 May;166(2):581–590. doi: 10.1128/jb.166.2.581-590.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PEEL D., QUAYLE J. R. Microbial growth on C1 compounds. I. Isolation and characterization of Pseudomonas AM 1. Biochem J. 1961 Dec;81:465–469. doi: 10.1042/bj0810465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruvkun G. B., Ausubel F. M. A general method for site-directed mutagenesis in prokaryotes. Nature. 1981 Jan 1;289(5793):85–88. doi: 10.1038/289085a0. [DOI] [PubMed] [Google Scholar]
- SAITO H., MIURA K. I. PREPARATION OF TRANSFORMING DEOXYRIBONUCLEIC ACID BY PHENOL TREATMENT. Biochim Biophys Acta. 1963 Aug 20;72:619–629. [PubMed] [Google Scholar]
- Salem A. R., Wagner C., Hacking A. J., Quayle J. R. The metabolism of lactate and pyruvate by Pseudomonas AM1. J Gen Microbiol. 1973 Jun;76(2):375–388. doi: 10.1099/00221287-76-2-375. [DOI] [PubMed] [Google Scholar]
- Taylor I. J., Anthony C. Acetyl-CoA production and utilization during growth of the facultative methylotroph Pseudomonas AM1 on ethanol, malonate and 3-hydroxybutyrate. J Gen Microbiol. 1976 Jul;95(1):134–143. doi: 10.1099/00221287-95-1-134. [DOI] [PubMed] [Google Scholar]
- Van Spanning R. J., Wansell C., Harms N., Oltmann L. F., Stouthamer A. H. Mutagenesis of the gene encoding cytochrome c550 of Paracoccus denitrificans and analysis of the resultant physiological effects. J Bacteriol. 1990 Feb;172(2):986–996. doi: 10.1128/jb.172.2.986-996.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Whitaker J. R., Granum P. E. An absolute method for protein determination based on difference in absorbance at 235 and 280 nm. Anal Biochem. 1980 Nov 15;109(1):156–159. doi: 10.1016/0003-2697(80)90024-x. [DOI] [PubMed] [Google Scholar]