Abstract
Immunization of adult macaques with live attenuated simian immunodeficiency viruses (SIVs) lacking the nef genes has been shown to protect against challenge with full-length pathogenic SIV. To test live attenuated virus vaccines for the first time in a natural host we have constructed a mutant SIV from African green monkeys (SIVagm) with a deletion of 125 bp in the nef gene (SIVagm3Δnef). This mutant showed moderately delayed in vitro replication in the T cell line MOLT-4/8 and in primary peripheral blood mononuclear cells from African green monkeys (Cercopithecus aetiops) and pig-tailed macaques (Macaca nemestrina) compared with cloned wild-type SIVagm3. In contrast, in vivo replication of SIVagm3Δnef in African green monkeys was severely impaired or undetectable and did not induce seroconversion. After challenge with wild-type SIVagm3 the SIVagm3Δnef preinoculated African green monkeys showed a memory antibody response that declined after week 2. In three of four African green monkeys the cell-associated virus load and in two of four African green monkeys the plasma virus load was dramatically decreased after the challenge compared with naive control animals. The remaining animal showed no evidence of productive challenge virus replication. This study demonstrates that a strong vaccine effect or protection in the SIVagm/African green monkey system is possible using a live attenuated vaccine in the absence of a productive infection and corresponding humoral immune response.
Simian immunodeficiency viruses (SIVs) are an essential model for assessing novel vaccine strategies for AIDS. Both SIVs and human immunodeficiency viruses (HIVs) use the CD4 molecule as initial receptor for entry into cells, and they contain a similar complement of auxiliary genes that appear to play similar roles in regulating virus replication (1, 2).
Nef, a regulatory gene of HIV and SIV located at the 3′ end of the genome, is a unique and conserved feature of the primate lentiviruses (3), which suggests an important role in the virus life cycle. Early studies indicated that Nef acts as a negative regulatory factor of virus replication and transcription (4–6), but later studies demonstrated a positive effect of Nef on the rate of viral replication (7–9). Indeed, nef-deficient mutants of SIV replicate slower in macaques in vivo than the corresponding nef-open wild-type viruses (10). By using such mutants as live attenuated vaccines, Daniel et al. (11) first demonstrated that prior infection (10) would fully protect from subsequent challenge with the pathogenic wild-type virus. Further deletions in the SIVmac vpr and NRE genes by Wyand et al. (12) to yield the highly attenuated SIVmac239Δ3 showed that it is possible to produce an effective vaccine strain with virtually no chance of reversion.
The protection against pathogenic challenge induced by live attenuated vaccines is by far the strongest yet achieved in the SIV model. However, the recent demonstration by Baba et al. (13) that SIVmac239Δ3, which is totally nonpathogenic in adult monkeys, grows to high titers, and causes AIDS in neonatal macaques has understandably increased concerns about the safety of live attenuated retroviral vaccines. Most researchers therefore use attenuated SIV as a tool to help understand the mechanisms of protection so that novel and safe vaccines that stimulate such an immunity can be designed.
In the SIVmac239Δ3 vaccine study mentioned above (12), the protection against wild-type SIV correlated with the strength of the replication of the live attenuated virus and with the development of neutralizing antibodies. In contrast, Clerici et al. (14) showed that macaques previously exposed intravenously to subinfectious doses of SIV did not develop SIV-specific antibodies but were nevertheless subsequently protected against an infectious intrarectal challenge of SIV 16 months later. As such monkeys demonstrated an SIV-specific cellular immune response at time of challenge, it was concluded that an undetectable or abortive infection had stimulated a protective T cell response.
In this study, African green monkeys were inoculated with SIVagm3Δnef, a mutant SIV from African green monkeys with a deletion of 125 bp from the nef gene, which was highly replication competent in vitro, but in vivo was highly attenuated to the point of being undetectable in most animals. Thirty-one weeks later all four African green monkeys, together with four naive controls, were challenged intravenously with full-length SIVagm3 (nef open). Compared with the control monkeys the SIVagm3Δnef preinoculated monkeys showed a drastically decreased virus load or were protected from challenge. These data demonstrate that vaccine protection can be achieved in the absence of detectable vaccine virus replication and humoral immune response.
MATERIALS AND METHODS
Cells.
MOLT-4/8 cells (15) were kindly donated by M. Hayami (Kyoto University). The C8166 cell line was obtained from the Medical Research Council AIDS Reagent Project (United Kingdom), the CEMss cell line from the AIDS Research and Reference Reagent Program of the National Institutes of Health, and the COS-7 cell line from the American Type Culture Collection. Peripheral blood mononuclear cells (PBMCs) were separated by Ficoll/Hypaque density gradient centrifugation of whole blood. Lymph node mononuclear cells (LNMCs) were obtained by Dounce homogenization and further purified by Ficoll/Hypaque density gradient centrifugation. All cells were maintained in RPMI 1640 medium supplemented with 10% (vol/vol) [or 20% (vol/vol) for PBMCs] fetal calf serum, l-glutamine, and antibiotics.
Construction of a Molecular SIVagm3Δnef Clone.
To introduce a deletion of 125 bp (8426–8550 bp) into the nef reading frame of SIVagm3 [SIVagm3 mc; GenBank accession no. M30931M30931], two fragments were generated by standard PCR with 50 ng of plasmid pSIVMB1 DNA (16) as template using the primers ECO-PLUS (5′-AGG GGG AAT TCT TCT ATT GTA AAA TGG ATT GG-3′), ECO-MINUS (5′-TAC CAT TTC TCT TCC TAA T-3′), SAL-PLUS (5′-TAG GAA GAG AAA TGG TAG CGG TGG ACT TTT CGC AC-3′), and SAL-MINUS (5′-CGG CAG TCG ACA AGC CTA CTC ACC AAG CCC T-3′), respectively. The two fragments were subsequently gel purified, assembled by PCR using the primer pair ECO-PLUS and SAL-MINUS, and inserted into the vector pUC18 (Pharmacia) to generate the plasmid pBES2.2. The presence of the deletion in the nef gene was verified by nucleotide sequencing. In parallel a SacI/EcoRI fragment was excised from pSIVMB1 and cloned into the vector pBluescript II KS (named pBES1.6; see Fig. 1). The EcoRI/SalI insert of pBES2.2 was introduced into pBES1.6 to generate a complete 3′-half of SIVagm3 carrying a 125-bp deletion in the nef gene (pBES3.8; Fig. 1B). To obtain replicating SIVagm3Δnef, the insert of pBES3.8 was ligated with a EcoNI/SacI fragment, which represents the 5′-half of the virus clone (Fig. 1B). The ligation reactions were purified and transfected into COS-7 cells. After 2 days, the cultures were overlaid with MOLT-4/8 cells. Cytopathic effects indicative of virus replication appeared within 1 week. DNA prepared from the infected MOLT-4/8 cells was amplified by PCR in the nef gene and sequenced to confirm the deletion.
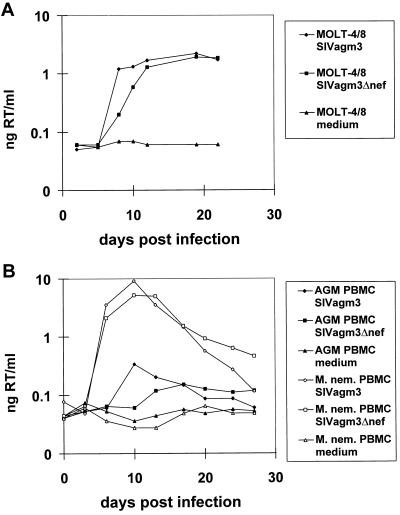
Figure 1.
(A) Growth kinetics of SIVagm3Δnef and of SIVagm3 (nef open) in MOLT-4/8 cells. MOLT-4/8 cells were infected in duplicate at a multiplicity of infection of 4 × 10−4 either with SIVagm3Δnef, SIVagm3 (nef open), or exposed to medium alone. Supernatants were assayed for RT activity. (B) Growth kinetics of SIVagm3Δnef and of SIVagm3 on phytohemagglutinin blasts of an SIVagm-negative African green monkey or a pig-tailed macaque. PBMCs were infected with a multiplicity of infection of 3 × 10−4 either with SIVagm3Δnef, SIVagm3 (nef open), or exposed to medium alone. Supernatants were assayed for RT activity. M. nem, Macaca nemestrina.
Host Range, Virus Stock Production, and Kinetics of SIVagm3Δnef.
SIVagm3Δnef was tested for virus replication in C8166, CEMss, and MOLT-4/8 cells, as well as in PBMCs from African green monkeys, cynomolgus monkeys (Macaca fascicularis), and pig-tailed macaques by virus-specific immunoperoxidase staining as well as by reverse transcriptase (RT) activity (Boehringer Mannheim).
First, stocks of SIVagm3Δnef and SIVagm3 (nef open) were produced in MOLT-4/8 cells yielding titers of 6.4 × 104 TCID50 and 8.1 × 104 TCID50, respectively. Both were used to infect MOLT-4/8 cells in duplicate at a multiplicity of infection of 4 × 10−4 and after 3 hr the cells were washed twice with PBS and resuspended in medium. Supernatants, taken at regular intervals (days 2–22), were tested for RT activity using a commercial kit (Boehringer Mannheim).
In addition, a PBMC grown stock of SIVagm3Δnef was produced for inoculation of African green monkeys. PBMCs from four seronegative African green monkeys were stimulated separately with phytohemagglutinin (54 μg/ml; Murex Diagnostics, Dartfort, U.K.) over a period of 3 days, washed once, and then infected overnight with SIVagm3Δnef. Supernatants were harvested on days 7, 10, and 14, passed through a 0.45-μM filter, aliquoted, and titrated in C8166 cells. The supernatant with the highest titer (day 7; 2369 TCID50/ml) was used for the subsequent in vitro and in vivo studies. The presence of the nef deletion was again confirmed by nef-specific PCR.
To determine the in vitro kinetics of PBMC grown SIVagm3Δnef, three cultures of 4 × 106 phytohemagglutinin-stimulated PBMCs from an SIVagm-negative African green monkey or pig-tailed macaque were infected at a multiplicity of infection of 3 × 10−4 with either the PBMC grown SIVagm3Δnef, PBMC grown SIVagm3/nef open, or with medium alone. After overnight incubation, the cultures were washed three times with PBS and supernatants were harvested every 3–4 days for measurement of RT activity.
Inoculation of African Green Monkeys with SIVagm3Δnef and Challenge with SIVagm3.
To determine the in vivo characteristics of the SIVagm3Δnef mutant, four SIV and simian T-lymphotropic virus negative African green monkeys (AGM 111, 112, 113, 115) were inoculated intravenously with 1000 TCID50 SIVagm3Δnef grown on African green monkey PBMCs. The infectivity of the SIVagm3Δnef inoculation stock was confirmed by adding 100 TCID50 to C8166 cells in quadruplicate, all of which became infected. Thirty-one weeks post-inoculation all four African green monkeys together with four naive African green monkeys were challenged with 500 MID50 of in vivo titrated SIVagm3 grown on African green monkey PBMCs (17). In a previous study, eight of eight African green monkeys inoculated with 100 MID50 of this stock became persistently infected.
Virus Reisolation and Measurement of Cell-Associated Virus Load.
For virus reisolation, PBMCs were cocultivated with 5 × 106 C8166 cells for 56 days and monitored for the presence of SIVagm replication by immunoperoxidase staining. The cell-associated virus load was determined by limiting dilution coculture where 5-fold dilutions of PBMCs, starting with 106 PBMCs, were cocultivated with 1 × 106 C8166 cells in triplicate in 24-well plates. After 21 days, the cells were tested for infection by immunoperoxidase staining. The number of infected cells per million PBMCs was then calculated with a Poisson distribution fitting model.
Measurement of Plasma Virus Load.
The plasma virus load was determined using a combination of the Product Enhanced Reverse Transkriptase (PERT) Assay (18) and the 5′ nuclease assay (19), the detailed protocol for which will be published elsewhere. Briefly, virus was pelleted from plasma, lysed, and incubated with template bacteriophage MS-2 RNA to allow synthesis of the corresponding cDNA fragment by viral RT. DNA was then PCR amplified using MS-2-specific primers in the presence of a probe corresponding to a sequence within the amplified region and labeled with rhodamine and 5′ FAM (6-carboxyl-fluorescein) at the 3′ and 5′ ends, respectively. Hybridization of the probe to amplified DNA and subsequent digestion by the 5′–3′nucleolytic activity of Taq polymerase abrogates the quenching effect of the 3′ rhodamine on the reporter 5′ FAM. The relative fluorescence of the rhodamine and reporter FAM is used to calculate, as described in ref. 20, the ratio of digested to intact probe (proportional to the amount of PCR product), and this value is used to calculate the amount of RT in the plasma sample by comparing with standards of known RT.
ELISA.
Titers of virus-specific antibodies were determined using standard ELISA protocols. Sera were titrated on ELISA plates coated with detergent-disrupted sucrose gradient-purified whole SIVagm. The antibody titer of each serum was calculated by extrapolating the line of best fit in the linear area of the dilution curve and determining the dilution at which this line intersected the cutoff (OD = 0.2).
Diagnostic PCR.
To distinguish between Nef-deficient SIVagm3 and full-length Nef (wild type) the following primer pairs were used in a diagnostic PCR: (i) SIVagm-env-EX1 8120+ (5′-GCA GAA AGA ATC TGG CAC AGC AGA GTG-3′) and SIVagm-nef-EX2 8668-(5′-CCT CAT GCA GGT CTA CTG GTA CCA-3′) and (ii) SIVagm-nef-IN1 8244+ (5′-ATG GGC TTG GGG AAC TCA AAG CCG-3′) and SIVagm-nef-IN2 8626-(5′-GTC GGA GTA ATA AAT CCC ATC CAG TCC-3′). PBMCs (105) were lysed in the presence of sodium dodecyl sulfate, proteinase K, and 2 μl of Gene Amp 10× PCR buffer (Applied Biosystems) for 3 hr at 50°C before inactivating the proteinase K for 10 min at 95°C. Standard nested PCR was used to amplify a 369/244 bp product spanning the site of the nef deletion. Reaction products were separated by size using 2% agarose gel electrophoresis and the correct size of the amplified SIVagm genome was verified by comparison with a 100-bp DNA standard marker (GIBCO/BRL), which allowed SIVagm3 nef full-length sequences (369 bp) to be distinguished from those of SIVagm3Δnef (244 bp).
RESULTS
SIVagm3Δnef Has a Similar Host Range and a Delayed in Vitro Replication in Human and Simian Cells.
SIVagm3Δnef was replication-competent in vitro in C8166, CEMss, and MOLT-4/8 cells, as well as in PBMCs from African green monkeys, cynomolgus monkeys, and pig-tailed macaques. In the human T cell line, MOLT-4/8, SIVagm3Δnef showed a slightly delayed replication kinetic during the first 12 days after infection compared with SIVagm3 (Fig. 1A). Similarly, the nef-deleted virus replicated slower in phytohemagglutinin-stimulated PBMCs from African green monkeys or pig-tailed macaques during the first 13–17 days, but yielded higher amounts of virus at later time points (Fig. 1B). Both viruses were able to replicate better by a factor of 1.5 logs in PBMCs from pig-tailed macaques than in African green monkey PBMCs. The results of the RT assays were confirmed by titration for live virus in C8166 cells (data not shown). The number of viable cells decreased over time, especially in the PBMC cultures infected with SIVagm3, possibly due to the initial high virus replication (data not shown), although there was no correlation between the titer of virus and cell viability.
Low to Absent Virus Load and Lack of Humoral Immune Response in the African Green Monkeys Immunized with SIVagm3Δnef.
It was only possible to reisolate virus from 1 × 106 PBMCs of one animal at one time point (AGM 113, week 3; Table 1). From week 4 on, reisolation was attempted from 0.3 to 1.7 × 107 PBMCs or 0.1 to 1 × 107 CD4+PBMCs without success. In the diagnostic PCR, proviral DNA could be detected in AGM 113 at week 3, agreeing with the virus reisolation at this time and in AGM 111 in weeks 21 and 31. In week 21, 5–7 × 106 LNMCs from each animal failed to show positive virus reisolation when cocultivated with C8166 indicator cells (Table 1), although proviral sequences could be detected in 2 × 105 LNMCs in AGM 112 and 113.
Table 1.
Virus reisolation and diagnostic PCR
| wpi* | Cell no. used for
|
AGM 111 | AGM 112 | AGM 113 | AGM 115 | AGM 158 | AGM 159 | AGM 160 | AGM 190 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Reisolation | PCR | |||||||||
| 0 | 1 × 106 PBMC | 2 × 105 PBMC | −(−) | −(−) | −(−) | −(−) | ||||
| 1 | 1 × 106 PBMC | 2 × 105 PBMC | −(−) | −(−) | −(−) | −(−) | ||||
| 2 | 1 × 106 PBMC | 2 × 105 PBMC | −(−) | −(−) | −(−) | −(−) | ||||
| 3 | 1 × 106 PBMC | 2 × 105 PBMC | −(−) | −(−) | +(Δnef) | −(−) | ||||
| 4 | 3 × 106 PBMC | 2 × 105 PBMC | −(−) | −(−) | −(−) | −(−) | ||||
| 6 | 1–2 × 106 CD4+ PBMC | ND | −(ND) | −(ND) | −(ND) | −(ND) | ||||
| 8 | 5–10 × 106 CD4+ PBMC | 2 × 105 CD4+ PBMC | −(−) | −(−) | −(−) | −(−) | ||||
| 11 | 1–2 × 106 CD4+ PBMC | 2 × 105 CD4+ PBMC | −(−) | −(−) | −(−) | −(−) | ||||
| 16 | 1 × 107 PBMC | 2 × 105 PBMC | −(−) | −(−) | −(−) | −(−) | ||||
| 21 | 0.7–1.7 × 107 PBMC | 2 × 105 PBMC | −(Δnef) | −(−) | −(−) | −(−) | ||||
| 5–7 × 106 LNMC | 2 × 105 LNMC | −(ND) | −(Δnef) | −(Δnef) | −(−) | |||||
| 25 | 0.5–1 × 107 PBMC | 2 × 105 PBMC | −(−) | −(−) | −(−) | −(−) | ||||
| 31 | 1–2 × 106 PBMC | 2 × 105 PBMC | −(Δnef) | −(−) | −(−) | −(−) | −(−) | −(−) | −(−) | −(−) |
| 32 | 1 × 106 PBMC | 2 × 105 PBMC | −(Δnef) | −(−) | −(−) | −(−) | −(−) | −(−) | −(−) | −(−) |
| 33 | 1 × 106 PBMC | 2 × 105 PBMC | −(−) | −(−) | +(−) | −(−) | +(wt) | +(−) | +(wt) | −(−) |
| 34 | 1 × 106 PBMC | 2 × 105 PBMC | −(−) | +(wt) | +(−) | −(−) | +(wt) | +(wt) | +(wt) | +(−) |
| 35 | 1 × 106 PBMC | 2 × 105 PBMC | −(−) | +(−) | +(−) | −(−) | +(wt) | +(wt) | +(wt) | +(−) |
| 37 | 1 × 106 PBMC | 2 × 105 PBMC | −(−) | +(−) | −(−) | −(−) | +(wt) | +(wt) | +(wt) | +(−) |
| 39 | 1 × 106 PBMC | 2 × 105 PBMC | +(−) | +(−) | −(−) | −(−) | +(wt) | +(wt) | −(wt) | −(−) |
| 43 | 1 × 106 PBMC | 2 × 105 PBMC | −(−) | +(−) | −(−) | −(−) | +(wt) | +(wt) | +(wt) | −(−) |
| 47 | 1 × 106 PBMC | 2 × 105 PBMC | +(wt) | +(−) | +(−) | −(wt) | +(wt) | −(wt) | +(wt) | −(−) |
| 51 | 1 × 106 PBMC | 2 × 105 PBMC | +(wt) | +(−) | +(−) | −(wt) | +(wt) | +(wt) | +(wt) | −(−) |
| 55 | 1 × 106 PBMC | 2 ×105 PBMC | +(wt) | +(−) | −(−) | −(wt) | +(wt) | ND (wt) | +(wt) | −(wt) |
Results of diagnostic PCR in brackets. Δnef, Δnef proviral sequence; wt, wild-type proviral sequence; ND, not determined.
wpi, weeks post immunization.
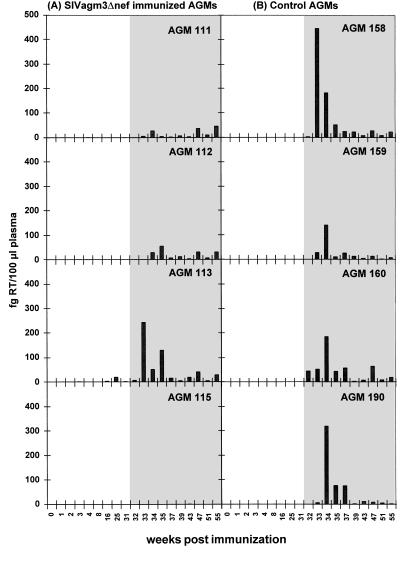
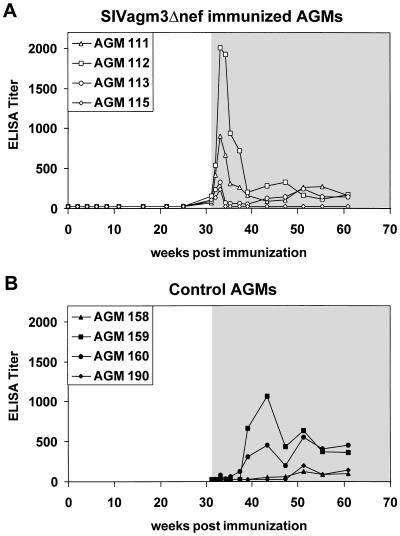
The highly sensitive PERT assay showed low RT activity (2.3–19.8 fg RT per 100 μl of plasma; Fig. 2A) in the plasma of AGM 113 at 16, 25, and 31 weeks. In the other three immunized African green monkeys the RT activity in the plasma remained below the detection limit of 1 fg per 100 μl of plasma. In none of the immunized African green monkeys could a humoral immune response before challenge be detected as measured by ELISA (Fig. 3A), immunoblot, or serum neutralization assay (data not shown).
Figure 2.
Plasma virus loads in the (A) SIVagm3Δnef immunized (AGM 111, 112, 113, 115) and the (B) control (AGM 158, 159, 160, 190) animals. The RT activities of the plasma (fg RT per 100 ml of plasma) were determined using the very sensitive PERT assay as described above. Open area, before challenge with SIVagm3; shaded area, after challenge with SIVagm3. Only AGM 113 exhibited prechallenge RT activity at 16, 25, and 31 weeks.
Figure 3.
Binding antibodies to whole SIVagm antigen in the (A) SIVagm3Δnef immunized (AGM 111, 112, 113, 115) and the (B) control (AGM 158, 159, 160, 190) animals. The binding antibodies were determined using a standard ELISA protocol. Open area, before challenge with SIVagm3; shaded area, after challenge with SIVagm3.
Vaccine Protection in the African Green Monkeys Challenged with SIVagm3.
Thirty-one weeks after inoculation with SIVagm3Δnef, African green monkeys 111, 112, 113, and 115, together with four naive controls were challenged intravenously with 500 MID50 SIVagm3. On the day of challenge all immunized animals were virus reisolation negative in the PBMCs (Table 1), although AGM 113 had a low level of RT activity in the plasma (2.3 fg RT per 100 μl of plasma; Fig. 2A) and proviral DNA could be detected by PCR in the PBMCs of AGM 111 (Table 1).
After challenge, the cell-associated virus load was dramatically decreased in the immunized animals compared with the controls (Table 2). In AGMs 112 and 113 only was a (low) peak at week 2 or 4 measurable (11 and 33 infected cells per million, respectively), whereas AGM 111 yielded only a low, sporadic virus reisolation from week 8 on and AGM 115 remained negative at all time points. In contrast, all four control animals showed a peak of virus replication in the first 4 weeks after the challenge ranging from 56 to 7029 infected cells per million. That the virus reisolated from the immunized and challenged animals was indeed the wild-type SIVagm3 was confirmed by diagnostic PCR.
Table 2.
SIVagm3/nef open infected PBMCs per million
| wpi* | SIVagm3Δnef inoculated
|
Controls
|
||||||
|---|---|---|---|---|---|---|---|---|
| AGM 111 | AGM 112 | AGM 113 | AGM 115 | AGM 158 | AGM 159 | AGM 160 | AGM 190 | |
| 31† | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 32 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 0 |
| 33 | 0 | 0 | 11 | 0 | 96 | 1 | 7,029 | 0 |
| 34 | 0 | 1 | 2 | 0 | 33 | 481 | 33 | 19 |
| 35 | 0 | 33 | 7 | 0 | 1 | 19 | 19 | 56 |
| 37 | 0 | 11 | 0 | 0 | 4 | 4 | 4 | 0 |
| 39 | 1 | 7 | 0 | 0 | 11 | 1 | 2 | 0 |
| 43 | 0 | 1 | 0 | 0 | 1 | 1 | 7 | 0 |
| 47 | 4 | ND | 1 | 0 | 1 | 2 | 33 | 0 |
| 51 | 1 | 1 | 1 | 0 | 4 | 1 | 33 | 0 |
| 55 | 1 | 7 | 0 | 0 | 1 | 0 | 7 | 0 |
| SUM | 7 | 61 | 22 | 0 | 152 | 510 | 7,171 | 75 |
ND, not determined.
wpi, Weeks post immunization.
Challenge with SIVagm3 in week 31.
Three of four control animals were persistently positive for proviral DNA, whereas the SIVagm3Δnef preinoculated monkeys were only sporadically positive for the full-length nef gene (Table 1).
In terms of the plasma virus load, the control African green monkeys showed a clear peak of virus in the plasma after inoculation at week 2 to 3, which ranged from 141 to 447 fg RT per 100 μl of plasma (Fig. 2B). In the immunized animals, two African green monkeys (111 and 112) showed a very low level RT activity in the plasma with the highest value of 47 fg RT per 100 μl of plasma in AGM 111 and of 56 in AGM 112 (Fig. 2A). In agreement with the cell-associated virus load AGM 115 did not show evidence of virus replication in the plasma. Only AGM 113 (which had been reisolation positive for the vaccine virus) showed a pattern of challenge virus replication similar to the controls.
Despite the lack of antibodies in response to SIVagm3Δnef inoculation, all immunized African green monkeys showed a memory antibody response after challenge. Peak antibody responses occurred at 2 weeks post challenge, at which point the infected control animals had yet to seroconvert. AGM 115, which did not show replication of the challenge virus, showed a weak memory antibody response and then returned to baseline levels. Neither the immunized nor the control monkeys developed neutralizing antibodies to SIVagm3 (data not shown).
In an effort to detect SIV-specific cellular immunity, the lymphoproliferative response to purified, inactivated SIVagm was measured using a [3H]thymidine incorporation assay. However, a stimulation index of more than 2 was measured in only two vaccinated monkeys (AGM 115 at 2 and 4 weeks post challenge and AGM 112 at week 4 weeks post challenge) and in two control monkeys (AGMs 159 and 190 at 4 weeks post challenge; data not shown). However, it is interesting to note that an 18% increase in the mean absolute number of CD8+ lymphocytes after challenge (n = 6) compared with the mean prechallenge values (n = 7) was seen in AGM 115 (immunized, protected animal; data not shown).
DISCUSSION
Infection with SIVagm is long established and endemic in the natural African green monkey host and, for reasons which are not entirely clear (21), does not result in the development of AIDS. Although the lack of pathogenesis precludes the use of disease development as an end point, the measurement of virus load allows vaccine protection or vaccine effects to be measured. Due to the often conflicting data obtained from studies using deletion mutants of SIVmac, we decided to produce and test in it’s natural host an SIVagm nef-deletion mutant to provide additional information from a different animal model.
Here we describe for the first time the in vitro and in vivo growth characteristics of a nef-deficient SIVagm mutant and it’s vaccine efficacy in African green monkeys. SIVagm3Δnef showed, during the first 2 weeks in culture, a delayed replication in MOLT-4/8 cells, African green monkey PBMCs, and PBMCs from pig-tailed macaques, compared with the wild-type virus. These differences, which have been observed for nef-deletion mutants of HIV-1 (22), may be due to the ability of Nef to act as a stimulator of T cells (23) or to down-regulate expression of CD4 (24). In only one report (25) have deletion mutants of SIVmac been shown to replicate slower in vitro, with most demonstrating replicative capacities similar to the parental viruses (10, 26, 27).
Compared with the SIVmac deletion mutants mentioned above, the replication of SIVagm3Δnef in vivo was very limited, with only one animal showing signs of productive virus replication in the PBMCs as well as in the plasma (Table 1, Fig. 2). In none of the inoculated animals could seroconversion to whole SIVagm antigen be detected. However, low level SIVagm3Δnef replication in all four African green monkeys was indicated by the memory immune response observed upon challenge (Fig. 3).
In each of the SIVmacΔnef (and SIVmacΔ3) experiments performed to date, protection has been accompanied by a significant antiviral humoral immune response. Indeed, protection in one study appeared to correlate with levels of antibody able to neutralize the primary challenge isolate (12). Clearly, in the case of SIVagm3Δnef the protection was unlikely to have been mediated by neutralizing antibodies as neither neutralizing nor even binding antibodies could be detected before challenge.
The protection afforded by deletion mutants of SIVmac also appears to be correlated to their replicative capacity. For example, the protective capacity of the triple deletion mutant SIVmac239Δ3 required more than 70 weeks to mature (12), whereas protection could be demonstrated using SIVmac32H pC8 (containing a four amino acid deletion in Nef) as early as 10 weeks after inoculation (28). Similarly, although deleted in only one gene (nef), SIVmac316Δnef had a more restricted growth capacity in vivo than SIVmac239Δ3 and gave a corresponding reduced degree of protection (12). In addition, we have recently shown that mutants of the relatively attenuated BK28 molecular clone of SIVmac251, containing large deletions in nef, protected rhesus macaques from pathogenic challenge in only one of eight cases, despite a long (42 week) period of “vaccination” (D. Binninger-Schinzel, unpublished work).
In contrast, the protection or vaccine effect demonstrated here using SIVagm3Δnef was not associated with detectable, productive infection of the inoculated African green monkeys. Indeed, the poorest protection in terms of virus loads after challenge was seen in the one animal in which productive infection could be demonstrated (AGM 113) and the best protection in the animal showing the least evidence of vaccine virus replication (AGM 115). These results are reminiscent of the studies using SIVmne in which pig-tailed macaques receiving “subinfectious” doses of the virus (as part of an in vivo titration) were subsequently resistant to challenge with infectious doses (14). In this case, although the animals were isolation, PCR, and antibody negative before challenge an SIV-specific cellular immune response could be demonstrated.
It is, however, possible that the success or failure of an attenuated SIV vaccine depends upon the replicative capacities of not only the vaccine virus but also of the challenge virus. This would be consistent with the idea that protection is due to competition for available target cells rather than the immune response induced. As the SIVagm3 molecular clone used for challenge in this study replicates to lower levels than the pathogenic, uncloned SIVmac stocks commonly used for SIVmac vaccine studies it is possible that the degree of vaccine virus replication required for protection is correspondingly lower.
It was not possible to reproducibly demonstrate an SIV-specific T cell lymphoproliferative response in the inoculated animals before (or after) challenge. Also, attempts to reproducibly demonstrate SIVagm-specific cytotoxic T lymphocytes in infected African green monkeys using autologous fibroblasts infected with vaccinia/SIV recombinants have so far failed, although this is possibly due to technical difficulties. The only indication of a T cell response in the immunized, protected animal (AGM 115) was an increase in the number of circulating CD8+ cells following challenge (data not shown). The basis (immunological or otherwise) for the protection or vaccine effect seen in this study therefore remains to be determined.
To summarize, the SIVagm3Δnef produced for this study, although exhibiting only marginally delayed replication kinetics in vitro, was highly attenuated in vivo. Despite the lack of seroconversion and evidence for productive virus replication, animals challenged with the full-length SIVagm3 molecular clone demonstrated drastically reduced virus loads compared with naive controls. This indicates that in this system at least protection was not dependent on high-level vaccine virus replication and corresponding humoral immune response. Rather, it is possible that the highly attenuated nature of the deletion mutant had stimulated a protective cellular immune response similar to that previously associated with subinfectious or abortive infections.
Acknowledgments
We thank Manuela Schütze, Tanja Kearns, Nicole Norley, and Anne Reckziegel for excellent technical assistance and Dr. Roland Plesker and Dr. Cheik Coulibaly for the veterinary work. The work was supported by Contract 1506/TG04 from the Federal Ministry of Health to R.K.
ABBREVIATIONS
- SIV
simian immunodeficiency virus
- PBMCs
peripheral blood mononuclear cells
- RT
reverse transcriptase
- AGM
African green monkey
- LNMCs
lymph node mononuclear cells
References
- 1.Desrosiers R C. Annu Rev Immunol. 1990;8:557–578. doi: 10.1146/annurev.iy.08.040190.003013. [DOI] [PubMed] [Google Scholar]
- 2.Gibbs J S, Desrosiers R C. In: Human Retroviruses: Frontiers in Molecular Biology. Cullen B R, editor. London: Oxford Univ. Press; 1993. pp. 137–158. [Google Scholar]
- 3.Myers G, Korber B, Berzofsky J A, Smith R F, Pavlakis G N, editors. Human Retroviruses and AIDS. Los Alamos Natl. Lab., Los Alamos, NM: Theoretical Biology and Biophysics Group; 1991. p. IB54. [Google Scholar]
- 4.Ahmad N, Venkatesan S. Science. 1988;241:1481–1485. doi: 10.1126/science.3262235. [DOI] [PubMed] [Google Scholar]
- 5.Cheng M C, Iannello P, Shaw K, Luciw P A, Levy J A. Science. 1989;246:1629–1632. doi: 10.1126/science.2531920. [DOI] [PubMed] [Google Scholar]
- 6.Niederman T M, Thielan B J, Ratner L. Proc Natl Acad Sci USA. 1989;86:1128–1132. doi: 10.1073/pnas.86.4.1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Ronde A, Klaver B, Keulen W, Smit L, Goudsmit J. Virology. 1992;188:391–395. doi: 10.1016/0042-6822(92)90772-h. [DOI] [PubMed] [Google Scholar]
- 8.Miller M D, Warmerdam M T, Gaston I, Greene W C, Feinberg M B. J Exp Med. 1994;179:101–113. doi: 10.1084/jem.179.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zazopulos E, Haseltine W A. Virology. 1993;194:20–27. doi: 10.1006/viro.1993.1230. [DOI] [PubMed] [Google Scholar]
- 10.Kestler H, Ringler D J, Mori K, Panicali D L, Sehgal P K, Daniel M D, Desrosiers R C. Cell. 1991;65:651–662. doi: 10.1016/0092-8674(91)90097-i. [DOI] [PubMed] [Google Scholar]
- 11.Daniel M D, Kirchhoff F, Czajak S C, Sehgal P K, Desrosiers R C. Science. 1992;258:1938–1941. doi: 10.1126/science.1470917. [DOI] [PubMed] [Google Scholar]
- 12.Wyand M S, Manson K H, Garcia M M, Montefiori D, Desrosiers R C. J Virol. 1996;70:3724–3733. doi: 10.1128/jvi.70.6.3724-3733.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baba T W, Jeong Y S, Pennick D, Bronson R, Greene M F, Ruprecht R M. Science. 1995;267:1820–1825. doi: 10.1126/science.7892606. [DOI] [PubMed] [Google Scholar]
- 14.Clerici M, Clark E A, Polacino P, Axberg I, Kuller L, Casey N I, Morton W R, Shearer G M, Benveniste R E. AIDS. 1994;8:1391–1395. doi: 10.1097/00002030-199410000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Kikukawa R, Koyanagi Y, Harada S, Kobayashi N, Hatanaka M, Yamamoto N. J Virol. 1986;57:1159–1162. doi: 10.1128/jvi.57.3.1159-1162.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baier M, Werner A, Cichutek K, Garber C, Mueller C, Kraus G, Ferdinand F J, Hartung S, Papas T S, Kurth R. J Virol. 1989;63:5119–5123. doi: 10.1128/jvi.63.12.5119-5123.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Siegel F, Kurth R, Norley S. J Acquired Immune Defic Syndr Hum Retrovirol. 1995;8:217–226. doi: 10.1097/00042560-199503010-00001. [DOI] [PubMed] [Google Scholar]
- 18.Pyra H, Boeni J, Schuepbach J. Proc Natl Acad Sci USA. 1994;91:1544–1548. doi: 10.1073/pnas.91.4.1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holland P M, Abramson R D, Watson R, Gelfand D H. Proc Natl Acad Sci USA. 1991;88:7276–7280. doi: 10.1073/pnas.88.16.7276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gerard C J, Arboleda M J, Solar G, Mulé J J, Kerr W G. Hum Gene Ther. 1996;7:343–354. doi: 10.1089/hum.1996.7.3-343. [DOI] [PubMed] [Google Scholar]
- 21.Kurth R, Norley S N. J Natl Inst Health Res. 1996;8:33–37. [Google Scholar]
- 22.Chowers M Y, Spina C A, Kwoh T J, Fitch N J, Richman D D, Guatelli J C. J Virol. 1994;68:2906–2914. doi: 10.1128/jvi.68.5.2906-2914.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cullen B R. Virology. 1994;205:1–6. doi: 10.1006/viro.1994.1613. [DOI] [PubMed] [Google Scholar]
- 24.Mariani R, Skowronski J. Proc Natl Acad Sci USA. 1993;90:5549–5553. doi: 10.1073/pnas.90.12.5549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Binninger D, Ennen J, Bonn D, Norley S G, Kurth R. J Virol. 1991;65:5237–5243. doi: 10.1128/jvi.65.10.5237-5243.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gibbs J S, Regier D A, Desrosiers R C. AIDS Res Hum Retroviruses. 1994;10:333–342. doi: 10.1089/aid.1994.10.333. [DOI] [PubMed] [Google Scholar]
- 27.Rud E W, Cranage M, Yon J, Quirk J, Ogilvie L, Cook N, Webster S, Dennis M, Clarke B E. J Gen Virol. 1994;75:529–543. doi: 10.1099/0022-1317-75-3-529. [DOI] [PubMed] [Google Scholar]
- 28.Norley S, Beer B, Binninger S D, Cosma C, Kurth R. Virology. 1996;219:195–205. doi: 10.1006/viro.1996.0237. [DOI] [PubMed] [Google Scholar]