Abstract
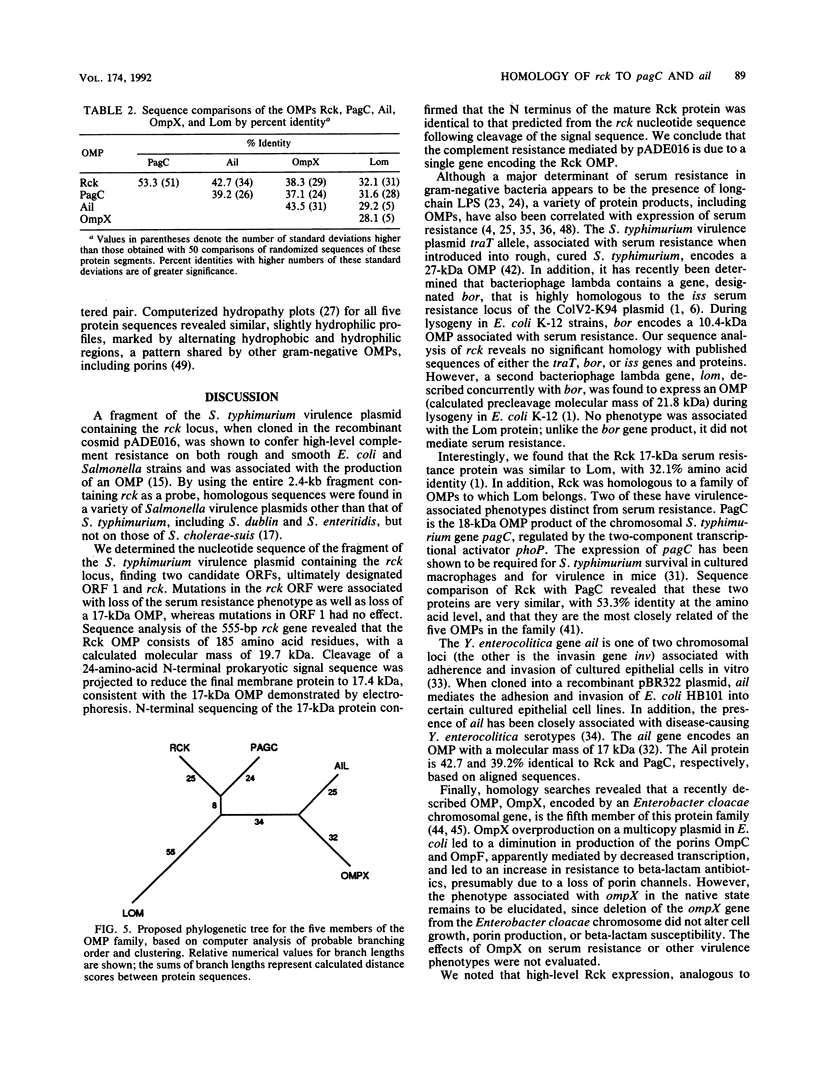
A fragment of the Salmonella typhimurium virulence plasmid containing the rck locus, when cloned in the recombinant cosmid pADE016, was shown previously to confer high-level complement resistance on both rough and smooth Escherichia coli, Salmonella minnesota, and S. typhimurium and was associated with the production of an outer membrane protein. We determined the nucleotide sequence of the fragment containing the rck locus. Mutations in the two major open reading frames confirmed that the complement resistance mediated by pADE016 was due to a single 555-bp rck gene encoding a 17-kDa outer membrane protein. Analysis of the rck gene revealed that the Rck outer membrane protein consisted of 185 amino acid residues, with a calculated postcleavage molecular mass of 17.4 kDa. Rck is homologous to a family of outer membrane proteins expressed in gram-negative bacteria, two of which have been associated with virulence-related phenotypes: PagC, required by S. typhimurium for survival in macrophages and for virulence in mice; and Ail, a product of the Yersinia enterocolitica chromosome capable of mediating bacterial adherence to and invasion of epithelial cell lines. Rck, most closely related to PagC, represents the third outer membrane protein in this five-member family with a distinct virulence-associated phenotype.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barondess J. J., Beckwith J. A bacterial virulence determinant encoded by lysogenic coliphage lambda. Nature. 1990 Aug 30;346(6287):871–874. doi: 10.1038/346871a0. [DOI] [PubMed] [Google Scholar]
- Beck E., Bremer E. Nucleotide sequence of the gene ompA coding the outer membrane protein II of Escherichia coli K-12. Nucleic Acids Res. 1980 Jul 11;8(13):3011–3027. doi: 10.1093/nar/8.13.3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beninger P. R., Chikami G., Tanabe K., Roudier C., Fierer J., Guiney D. G. Physical and genetic mapping of the Salmonella dublin virulence plasmid pSDL2. Relationship to plasmids from other Salmonella strains. J Clin Invest. 1988 May;81(5):1341–1347. doi: 10.1172/JCI113461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaser M. J., Smith P. F., Hopkins J. A., Heinzer I., Bryner J. H., Wang W. L. Pathogenesis of Campylobacter fetus infections: serum resistance associated with high-molecular-weight surface proteins. J Infect Dis. 1987 Apr;155(4):696–706. doi: 10.1093/infdis/155.4.696. [DOI] [PubMed] [Google Scholar]
- Braun G., Cole S. T. DNA sequence analysis of the Serratia marcescens ompA gene: implications for the organisation of an enterobacterial outer membrane protein. Mol Gen Genet. 1984;195(1-2):321–328. doi: 10.1007/BF00332766. [DOI] [PubMed] [Google Scholar]
- Chuba P. J., Leon M. A., Banerjee A., Palchaudhuri S. Cloning and DNA sequence of plasmid determinant iss, coding for increased serum survival and surface exclusion, which has homology with lambda DNA. Mol Gen Genet. 1989 Apr;216(2-3):287–292. doi: 10.1007/BF00334367. [DOI] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C., Hsu L. Nonchromosomal antibiotic resistance in bacteria: genetic transformation of Escherichia coli by R-factor DNA. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2110–2114. doi: 10.1073/pnas.69.8.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corpet F. Multiple sequence alignment with hierarchical clustering. Nucleic Acids Res. 1988 Nov 25;16(22):10881–10890. doi: 10.1093/nar/16.22.10881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagert M., Ehrlich S. D. Prolonged incubation in calcium chloride improves the competence of Escherichia coli cells. Gene. 1979 May;6(1):23–28. doi: 10.1016/0378-1119(79)90082-9. [DOI] [PubMed] [Google Scholar]
- Davis C. E., Freedman S. D., Douglas H., Braude A. I. Analysis of sugars in bacterial endotoxins by gas-liquid chromatography. Anal Biochem. 1969 Apr 4;28(1):243–256. doi: 10.1016/0003-2697(69)90175-4. [DOI] [PubMed] [Google Scholar]
- Doolittle R. F., Feng D. F. Nearest neighbor procedure for relating progressively aligned amino acid sequences. Methods Enzymol. 1990;183:659–669. doi: 10.1016/0076-6879(90)83043-9. [DOI] [PubMed] [Google Scholar]
- Feng D. F., Doolittle R. F. Progressive alignment and phylogenetic tree construction of protein sequences. Methods Enzymol. 1990;183:375–387. doi: 10.1016/0076-6879(90)83025-5. [DOI] [PubMed] [Google Scholar]
- Guiney D. G., Hasegawa P., Davis C. E. Plasmid transfer from Escherichia coli to Bacteroides fragilis: differential expression of antibiotic resistance phenotypes. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7203–7206. doi: 10.1073/pnas.81.22.7203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulig P. A. Virulence plasmids of Salmonella typhimurium and other salmonellae. Microb Pathog. 1990 Jan;8(1):3–11. doi: 10.1016/0882-4010(90)90003-9. [DOI] [PubMed] [Google Scholar]
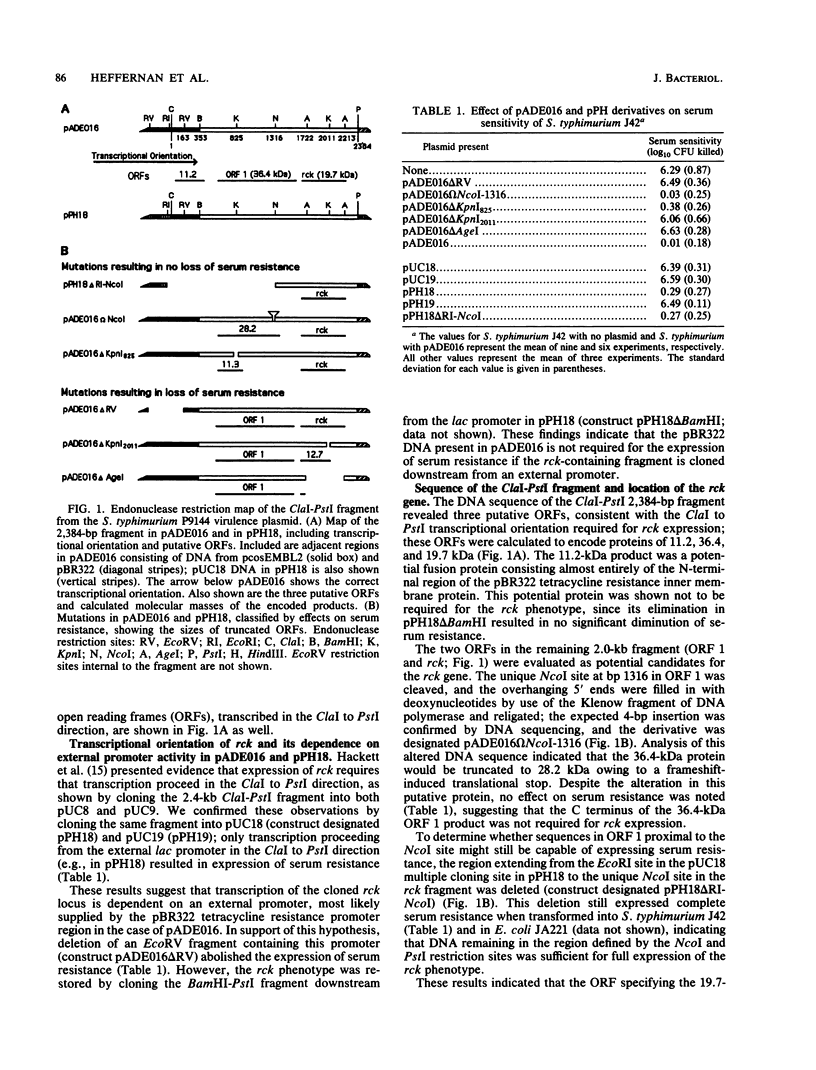
- Hackett J., Wyk P., Reeves P., Mathan V. Mediation of serum resistance in Salmonella typhimurium by an 11-kilodalton polypeptide encoded by the cryptic plasmid. J Infect Dis. 1987 Mar;155(3):540–549. doi: 10.1093/infdis/155.3.540. [DOI] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Hershfield V., Boyer H. W., Yanofsky C., Lovett M. A., Helinski D. R. Plasmid ColEl as a molecular vehicle for cloning and amplification of DNA. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3455–3459. doi: 10.1073/pnas.71.9.3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewick R. M., Hunkapiller M. W., Hood L. E., Dreyer W. J. A gas-liquid solid phase peptide and protein sequenator. J Biol Chem. 1981 Aug 10;256(15):7990–7997. [PubMed] [Google Scholar]
- Inokuchi K., Mutoh N., Matsuyama S., Mizushima S. Primary structure of the ompF gene that codes for a major outer membrane protein of Escherichia coli K-12. Nucleic Acids Res. 1982 Nov 11;10(21):6957–6968. doi: 10.1093/nar/10.21.6957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jap B. K., Walian P. J. Biophysics of the structure and function of porins. Q Rev Biophys. 1990 Nov;23(4):367–403. doi: 10.1017/s003358350000559x. [DOI] [PubMed] [Google Scholar]
- Joiner K. A. Complement evasion by bacteria and parasites. Annu Rev Microbiol. 1988;42:201–230. doi: 10.1146/annurev.mi.42.100188.001221. [DOI] [PubMed] [Google Scholar]
- Joiner K. A., Hammer C. H., Brown E. J., Cole R. J., Frank M. M. Studies on the mechanism of bacterial resistance to complement-mediated killing. I. Terminal complement components are deposited and released from Salmonella minnesota S218 without causing bacterial death. J Exp Med. 1982 Mar 1;155(3):797–808. doi: 10.1084/jem.155.3.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joiner K. A., Hammer C. H., Brown E. J., Frank M. M. Studies on the mechanism of bacterial resistance to complement-mediated killing. II. C8 and C9 release C5b67 from the surface of Salmonella minnesota S218 because the terminal complex does not insert into the bacterial outer membrane. J Exp Med. 1982 Mar 1;155(3):809–819. doi: 10.1084/jem.155.3.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joiner K. A., Warren K. A., Brown E. J., Swanson J., Frank M. M. Studies on the mechanism of bacterial resistance to complement-mediated killing. IV. C5b-9 forms high molecular weight complexes with bacterial outer membrane constituents on serum-resistant but not on serum-sensitive Neisseria gonorrhoeae. J Immunol. 1983 Sep;131(3):1443–1451. [PubMed] [Google Scholar]
- Kawahara K., Hamaoka T., Suzuki S., Nakamura M., Murayama S. Y., Arai T., Terakado N., Danbara H. Lipopolysaccharide alteration mediated by the virulence plasmid of Salmonella. Microb Pathog. 1989 Sep;7(3):195–202. doi: 10.1016/0882-4010(89)90055-7. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- McCutchan J. A., Levine S., Braude A. I. Influence of colony type on susceptibility of gonococci to killing by human serum. J Immunol. 1976 Jun;116(6):1652–1655. [PubMed] [Google Scholar]
- Medof M. E., Lublin D. M., Holers V. M., Ayers D. J., Getty R. R., Leykam J. F., Atkinson J. P., Tykocinski M. L. Cloning and characterization of cDNAs encoding the complete sequence of decay-accelerating factor of human complement. Proc Natl Acad Sci U S A. 1987 Apr;84(7):2007–2011. doi: 10.1073/pnas.84.7.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller S. I., Kukral A. M., Mekalanos J. J. A two-component regulatory system (phoP phoQ) controls Salmonella typhimurium virulence. Proc Natl Acad Sci U S A. 1989 Jul;86(13):5054–5058. doi: 10.1073/pnas.86.13.5054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller V. L., Bliska J. B., Falkow S. Nucleotide sequence of the Yersinia enterocolitica ail gene and characterization of the Ail protein product. J Bacteriol. 1990 Feb;172(2):1062–1069. doi: 10.1128/jb.172.2.1062-1069.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller V. L., Falkow S. Evidence for two genetic loci in Yersinia enterocolitica that can promote invasion of epithelial cells. Infect Immun. 1988 May;56(5):1242–1248. doi: 10.1128/iai.56.5.1242-1248.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller V. L., Farmer J. J., 3rd, Hill W. E., Falkow S. The ail locus is found uniquely in Yersinia enterocolitica serotypes commonly associated with disease. Infect Immun. 1989 Jan;57(1):121–131. doi: 10.1128/iai.57.1.121-131.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll A., Manning P. A., Timmis K. N. Plasmid-determined resistance to serum bactericidal activity: a major outer membrane protein, the traT gene product, is responsible for plasmid-specified serum resistance in Escherichia coli. Infect Immun. 1980 May;28(2):359–367. doi: 10.1128/iai.28.2.359-367.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munn C. B., Ishiguro E. E., Kay W. W., Trust T. J. Role of surface components in serum resistance of virulent Aeromonas salmonicida. Infect Immun. 1982 Jun;36(3):1069–1075. doi: 10.1128/iai.36.3.1069-1075.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada H., Nagami Y., Takahashi K., Okada N., Hideshima T., Takizawa H., Kondo J. 20 KDa homologous restriction factor of complement resembles T cell activating protein. Biochem Biophys Res Commun. 1989 Aug 15;162(3):1553–1559. doi: 10.1016/0006-291x(89)90852-8. [DOI] [PubMed] [Google Scholar]
- Overbeeke N., Bergmans H., van Mansfeld F., Lugtenberg B. Complete nucleotide sequence of phoE, the structural gene for the phosphate limitation inducible outer membrane pore protein of Escherichia coli K12. J Mol Biol. 1983 Feb 5;163(4):513–532. doi: 10.1016/0022-2836(83)90110-9. [DOI] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portnoy D. A., Wolf-Watz H., Bolin I., Beeder A. B., Falkow S. Characterization of common virulence plasmids in Yersinia species and their role in the expression of outer membrane proteins. Infect Immun. 1984 Jan;43(1):108–114. doi: 10.1128/iai.43.1.108-114.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulkkinen W. S., Miller S. I. A Salmonella typhimurium virulence protein is similar to a Yersinia enterocolitica invasion protein and a bacteriophage lambda outer membrane protein. J Bacteriol. 1991 Jan;173(1):86–93. doi: 10.1128/jb.173.1.86-93.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhen M., Sukupolvi S. The role of the traT gene of the Salmonella typhimurium virulence plasmid for serum resistance and growth within liver macrophages. Microb Pathog. 1988 Oct;5(4):275–285. doi: 10.1016/0882-4010(88)90100-3. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoorvogel J., van Bussel M. J., Tommassen J., van de Klundert J. A. Molecular characterization of an Enterobacter cloacae outer membrane protein (OmpX). J Bacteriol. 1991 Jan;173(1):156–160. doi: 10.1128/jb.173.1.156-160.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoorvogel J., van Bussel M. J., van de Klundert J. A. Biological characterization of an Enterobacter cloacae outer membrane protein (OmpX). J Bacteriol. 1991 Jan;173(1):161–167. doi: 10.1128/jb.173.1.161-167.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor P. W. Bactericidal and bacteriolytic activity of serum against gram-negative bacteria. Microbiol Rev. 1983 Mar;47(1):46–83. doi: 10.1128/mr.47.1.46-83.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas C. M. Complementation analysis of replication and maintenance functions of broad host range plasmids RK2 and RP1. Plasmid. 1981 May;5(3):277–291. doi: 10.1016/0147-619x(81)90005-6. [DOI] [PubMed] [Google Scholar]
- Timmis K. N., Boulnois G. J., Bitter-Suermann D., Cabello F. C. Surface components of Escherichia coli that mediate resistance to the bactericidal activities of serum and phagocytes. Curr Top Microbiol Immunol. 1985;118:197–218. doi: 10.1007/978-3-642-70586-1_11. [DOI] [PubMed] [Google Scholar]
- Vandenbosch J. L., Kurlandsky D. R., Urdangaray R., Jones G. W. Evidence of coordinate regulation of virulence in Salmonella typhimurium involving the rsk element of the 95-kilobase plasmid. Infect Immun. 1989 Aug;57(8):2566–2568. doi: 10.1128/iai.57.8.2566-2568.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenbosch J. L., Rabert D. K., Kurlandsky D. R., Jones G. W. Sequence analysis of rsk, a portion of the 95-kilobase plasmid of Salmonella typhimurium associated with resistance to the bactericidal activity of serum. Infect Immun. 1989 Mar;57(3):850–857. doi: 10.1128/iai.57.3.850-857.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- von Heijne G. Transcending the impenetrable: how proteins come to terms with membranes. Biochim Biophys Acta. 1988 Jun 9;947(2):307–333. doi: 10.1016/0304-4157(88)90013-5. [DOI] [PubMed] [Google Scholar]