Abstract
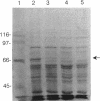
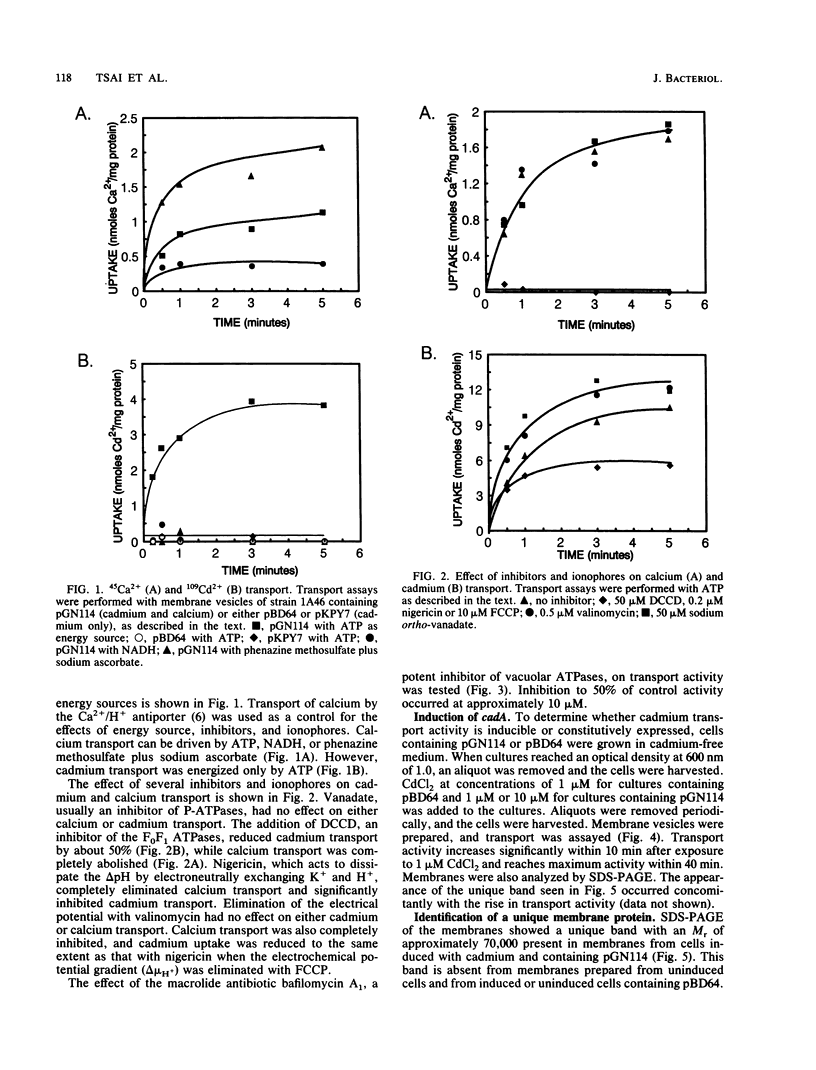
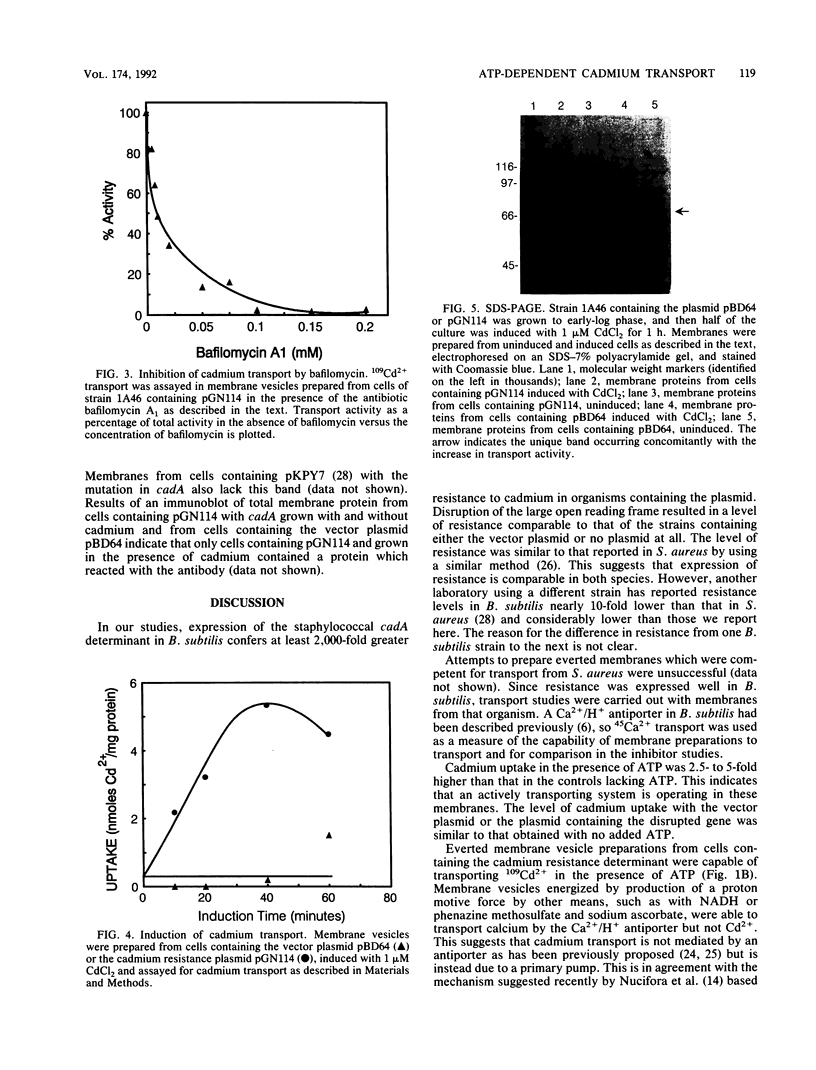
Resistance to cadmium conferred by the staphylococcal plasmid pI258 occurs by means of energy-dependent efflux, resulting in decreased intracellular accumulation of cadmium. Recent sequence information suggested that efflux is mediated by a P-type ATPase. The cadA gene was previously expressed in Bacillus subtilis, conferring resistance to cadmium. Everted membrane vesicles were prepared from B. subtilis cells harboring either a plasmid containing the cadA system or the vector plasmid alone. 109Cd2+ transport into the everted membranes was measured in the presence of various energy sources. Cadmium transport was detected only in the presence of ATP as an energy source. The production of an electrochemical proton gradient (delta mu H+) by using NADH or phenazine methosulfate plus ascorbate was not able to drive transport. Reagents which dissipate delta pH abolished calcium transport due to the Ca2+/H+ antiporter but only partially inhibited cadmium transport. Inhibition of transport by the antibiotic bafilomycin A1 occurred at concentrations comparable to those which inhibit P-type ATPases. A band corresponding to the cadA gene product was identified on sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and antibodies to the protein were prepared.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambudkar S. V., Lynn A. R., Maloney P. C., Rosen B. P. Reconstitution of ATP-dependent calcium transport from streptococci. J Biol Chem. 1986 Nov 25;261(33):15596–15600. [PubMed] [Google Scholar]
- Ames G. F. Resolution of bacterial proteins by polyacrylamide gel electrophoresis on slabs. Membrane, soluble, and periplasmic fractions. J Biol Chem. 1974 Jan 25;249(2):634–644. [PubMed] [Google Scholar]
- Bensadoun A., Weinstein D. Assay of proteins in the presence of interfering materials. Anal Biochem. 1976 Jan;70(1):241–250. doi: 10.1016/s0003-2697(76)80064-4. [DOI] [PubMed] [Google Scholar]
- Bowman B. J., Slayman C. W. The effects of vanadate on the plasma membrane ATPase of Neurospora crassa. J Biol Chem. 1979 Apr 25;254(8):2928–2934. [PubMed] [Google Scholar]
- Bowman E. J., Siebers A., Altendorf K. Bafilomycins: a class of inhibitors of membrane ATPases from microorganisms, animal cells, and plant cells. Proc Natl Acad Sci U S A. 1988 Nov;85(21):7972–7976. doi: 10.1073/pnas.85.21.7972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fürst P., Solioz M. Formation of a beta-aspartyl phosphate intermediate by the vanadate-sensitive ATPase of Streptococcus faecalis. J Biol Chem. 1985 Jan 10;260(1):50–52. [PubMed] [Google Scholar]
- Houng H. S., Lynn A. R., Rosen B. P. ATP-driven calcium transport in membrane vesicles of Streptococcus sanguis. J Bacteriol. 1986 Nov;168(2):1040–1044. doi: 10.1128/jb.168.2.1040-1044.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephson L., Cantley L. C., Jr Isolation of a potent (Na-K)ATPase inhibitor from striated muscle. Biochemistry. 1977 Oct 18;16(21):4572–4578. doi: 10.1021/bi00640a006. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Novick R. P., Roth C. Plasmid-linked resistance to inorganic salts in Staphylococcus aureus. J Bacteriol. 1968 Apr;95(4):1335–1342. doi: 10.1128/jb.95.4.1335-1342.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nucifora G., Chu L., Misra T. K., Silver S. Cadmium resistance from Staphylococcus aureus plasmid pI258 cadA gene results from a cadmium-efflux ATPase. Proc Natl Acad Sci U S A. 1989 May;86(10):3544–3548. doi: 10.1073/pnas.86.10.3544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neal S. G., Rhoads D. B., Racker E. Vanadate inhibition of sarcoplasmic reticulum Ca2+-ATPase and other ATPases. Biochem Biophys Res Commun. 1979 Aug 13;89(3):845–850. doi: 10.1016/0006-291x(79)91855-2. [DOI] [PubMed] [Google Scholar]
- Perry R. D., Silver S. Cadmium and manganese transport in Staphylococcus aureus membrane vesicles. J Bacteriol. 1982 May;150(2):973–976. doi: 10.1128/jb.150.2.973-976.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Schaffner W., Weissmann C. A rapid, sensitive, and specific method for the determination of protein in dilute solution. Anal Biochem. 1973 Dec;56(2):502–514. doi: 10.1016/0003-2697(73)90217-0. [DOI] [PubMed] [Google Scholar]
- Silver S., Nucifora G., Chu L., Misra T. K. Bacterial resistance ATPases: primary pumps for exporting toxic cations and anions. Trends Biochem Sci. 1989 Feb;14(2):76–80. doi: 10.1016/0968-0004(89)90048-0. [DOI] [PubMed] [Google Scholar]
- Smith I., Paress P., Cabane K., Dubnau E. Genetics and physiology of the rel system of Bacillus subtilis. Mol Gen Genet. 1980;178(2):271–279. doi: 10.1007/BF00270472. [DOI] [PubMed] [Google Scholar]
- Smith K., Novick R. P. Genetic studies on plasmid-linked cadmium resistance in Staphylococcus aureus. J Bacteriol. 1972 Nov;112(2):761–772. doi: 10.1128/jb.112.2.761-772.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tynecka Z., Gos Z., Zajac J. Energy-dependent efflux of cadmium coded by a plasmid resistance determinant in Staphylococcus aureus. J Bacteriol. 1981 Aug;147(2):313–319. doi: 10.1128/jb.147.2.313-319.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tynecka Z., Gos Z., Zajac J. Reduced cadmium transport determined by a resistance plasmid in Staphylococcus aureus. J Bacteriol. 1981 Aug;147(2):305–312. doi: 10.1128/jb.147.2.305-312.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tynecka Z., Skwarek T., Malm A. Anaerobic 109Cd accumulation by cadmium-resistant and -sensitive Staphylococcus aureus. FEMS Microbiol Lett. 1990 May;57(1-2):159–164. doi: 10.1016/0378-1097(90)90431-o. [DOI] [PubMed] [Google Scholar]
- Weiss A. A., Silver S., Kinscherf T. G. Cation transport alteration associated with plasmid-determined resistance to cadmium in Staphylococcus aureus. Antimicrob Agents Chemother. 1978 Dec;14(6):856–865. doi: 10.1128/aac.14.6.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon K. P., Misra T. K., Silver S. Regulation of the cadA cadmium resistance determinant of Staphylococcus aureus plasmid pI258. J Bacteriol. 1991 Dec;173(23):7643–7649. doi: 10.1128/jb.173.23.7643-7649.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon K. P., Silver S. A second gene in the Staphylococcus aureus cadA cadmium resistance determinant of plasmid pI258. J Bacteriol. 1991 Dec;173(23):7636–7642. doi: 10.1128/jb.173.23.7636-7642.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vrij W., Bulthuis R., Postma E., Konings W. N. Calcium transport in membrane vesicles of Bacillus subtilis. J Bacteriol. 1985 Dec;164(3):1294–1300. doi: 10.1128/jb.164.3.1294-1300.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]