Abstract
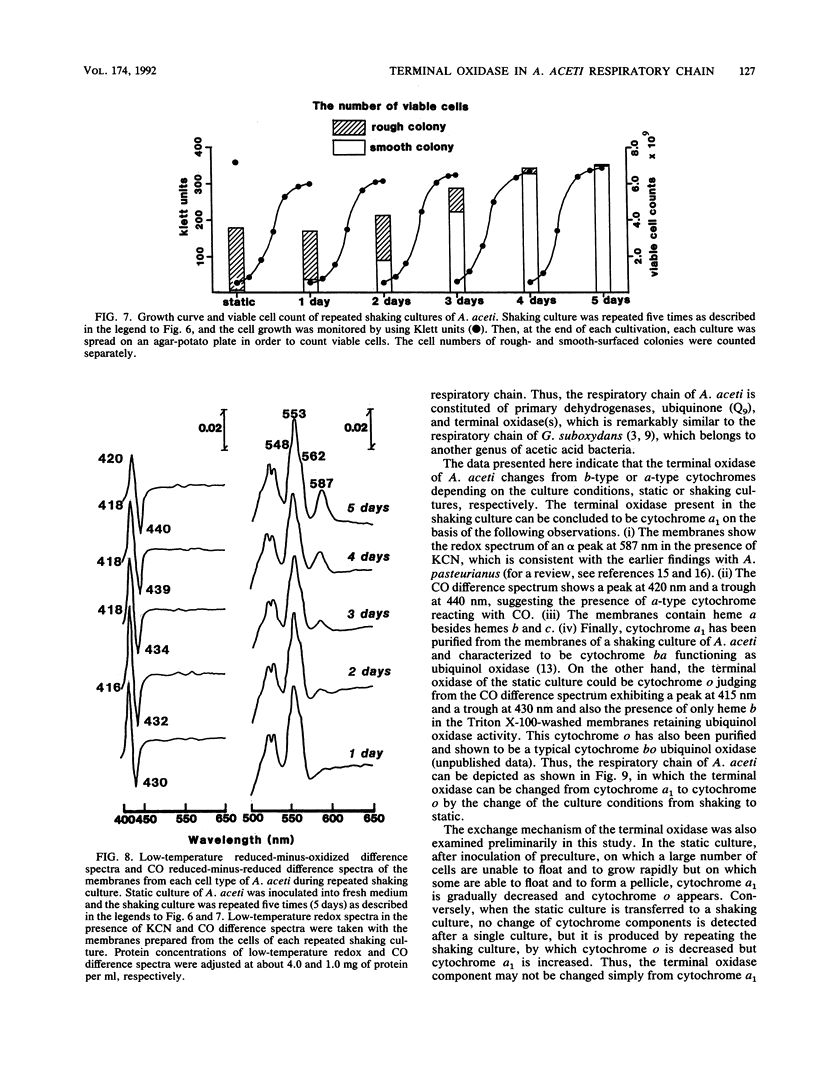
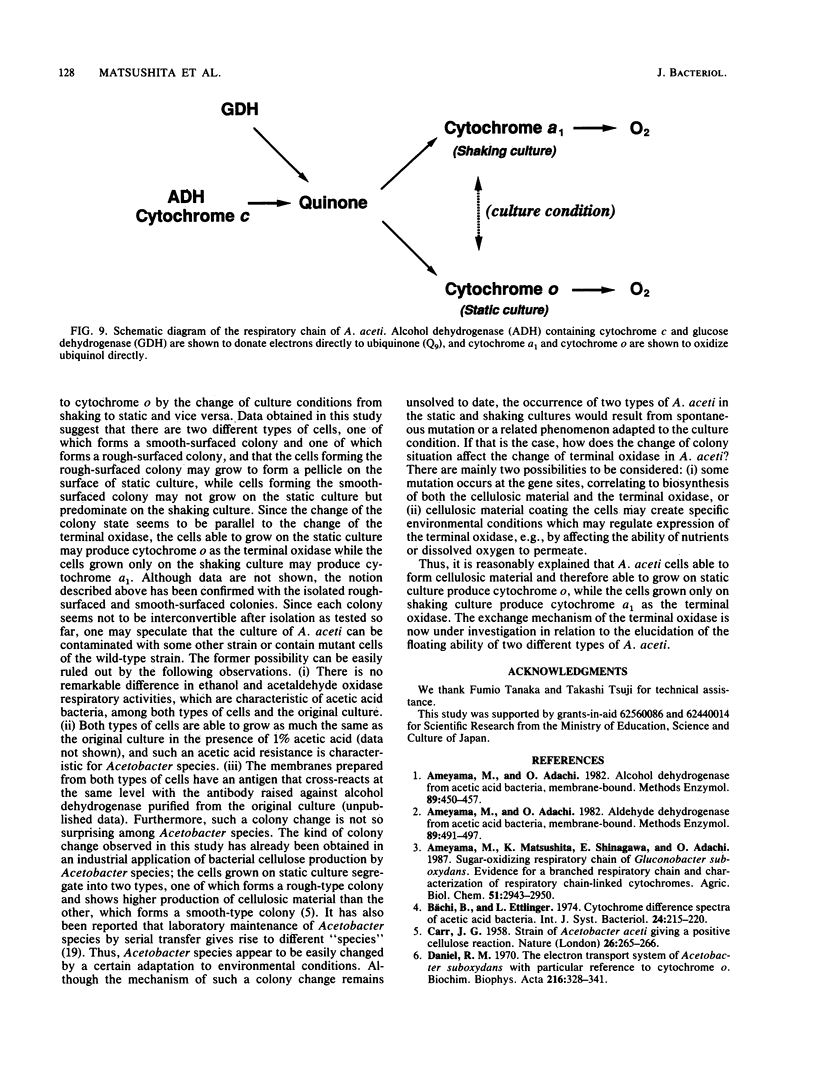
Acetobacter aceti has an ability to grow under two different culture conditions, on shaking submerged cultures and on static pellicle-forming cultures. The respiratory chains of A. aceti grown on shaking and static cultures were compared, especially with respect to the terminal oxidase. Little difference was detected in several oxidase activities and in cytochrome b and c contents between the respiratory chains of both types of cells. Furthermore, the results obtained here suggested that the respiratory chains consist of primary dehydrogenases, ubiquinone, and terminal ubiquinol oxidase, regardless of the culture conditions. There was a remarkable difference, however, in the terminal oxidase, which is cytochrome a1 in cells in shaking culture but cytochrome o in cells grown statically. Change of the culture condition from shaking to static caused a change in the terminal oxidase from cytochrome a1 to cytochrome o, which is concomitant with an increase of pellicle on the surface of the static culture. In contrast, reappearance of cytochrome a1 in A. aceti was attained only after serial successive shaking cultures of an original static culture; cytochrome a1 predominated after the culture was repeated five times. In the culture of A. aceti, two different types of cells were observed; one forms a rough-surfaced colony, and the other forms a smooth-surfaced colony. Cells of the former type predominated in the static culture, while the cells of the latter type predominated in the shaking culture. Thus, data suggest that a change of the culture conditions, from static to shaking or vice versa, results in a change of the cell type, which may be related to the change in the terminal oxidase from cytochrome a1 to cytochrome o in A. aceti.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CARR J. G. A strain of Acetobacter aceti giving a positive cellulose reaction. Nature. 1958 Jul 26;182(4630):265–266. doi: 10.1038/182265b0. [DOI] [PubMed] [Google Scholar]
- Daniel R. M. The electron transport system of Acetobacter suboxydans with particular reference to cytochrome. Biochim Biophys Acta. 1970 Sep 1;216(2):328–341. doi: 10.1016/0005-2728(70)90224-0. [DOI] [PubMed] [Google Scholar]
- Dulley J. R., Grieve P. A. A simple technique for eliminating interference by detergents in the Lowry method of protein determination. Anal Biochem. 1975 Mar;64(1):136–141. doi: 10.1016/0003-2697(75)90415-7. [DOI] [PubMed] [Google Scholar]
- Matsushita K., Ameyama M. D-Glucose dehydrogenase from Pseudomonas fluorescens, membrane-bound. Methods Enzymol. 1982;89(Pt 500):149–154. doi: 10.1016/s0076-6879(82)89026-5. [DOI] [PubMed] [Google Scholar]
- Matsushita K., Nagatani Y., Shinagawa E., Adachi O., Ameyama M. Reconstitution of the ethanol oxidase respiratory chain in membranes of quinoprotein alcohol dehydrogenase-deficient Gluconobacter suboxydans subsp. alpha strains. J Bacteriol. 1991 Jun;173(11):3440–3445. doi: 10.1128/jb.173.11.3440-3445.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushita K., Shinagawa E., Adachi O., Ameyama M. Cytochrome a1 of acetobacter aceti is a cytochrome ba functioning as ubiquinol oxidase. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9863–9867. doi: 10.1073/pnas.87.24.9863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushita K., Shinagawa E., Adachi O., Ameyama M. Reactivity with ubiquinone of quinoprotein D-glucose dehydrogenase from Gluconobacter suboxydans. J Biochem. 1989 Apr;105(4):633–637. doi: 10.1093/oxfordjournals.jbchem.a122716. [DOI] [PubMed] [Google Scholar]
- Poole R. K. Bacterial cytochrome oxidases. A structurally and functionally diverse group of electron-transfer proteins. Biochim Biophys Acta. 1983 Sep 15;726(3):205–243. doi: 10.1016/0304-4173(83)90006-x. [DOI] [PubMed] [Google Scholar]
- Ross P., Mayer R., Benziman M. Cellulose biosynthesis and function in bacteria. Microbiol Rev. 1991 Mar;55(1):35–58. doi: 10.1128/mr.55.1.35-58.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]