Abstract
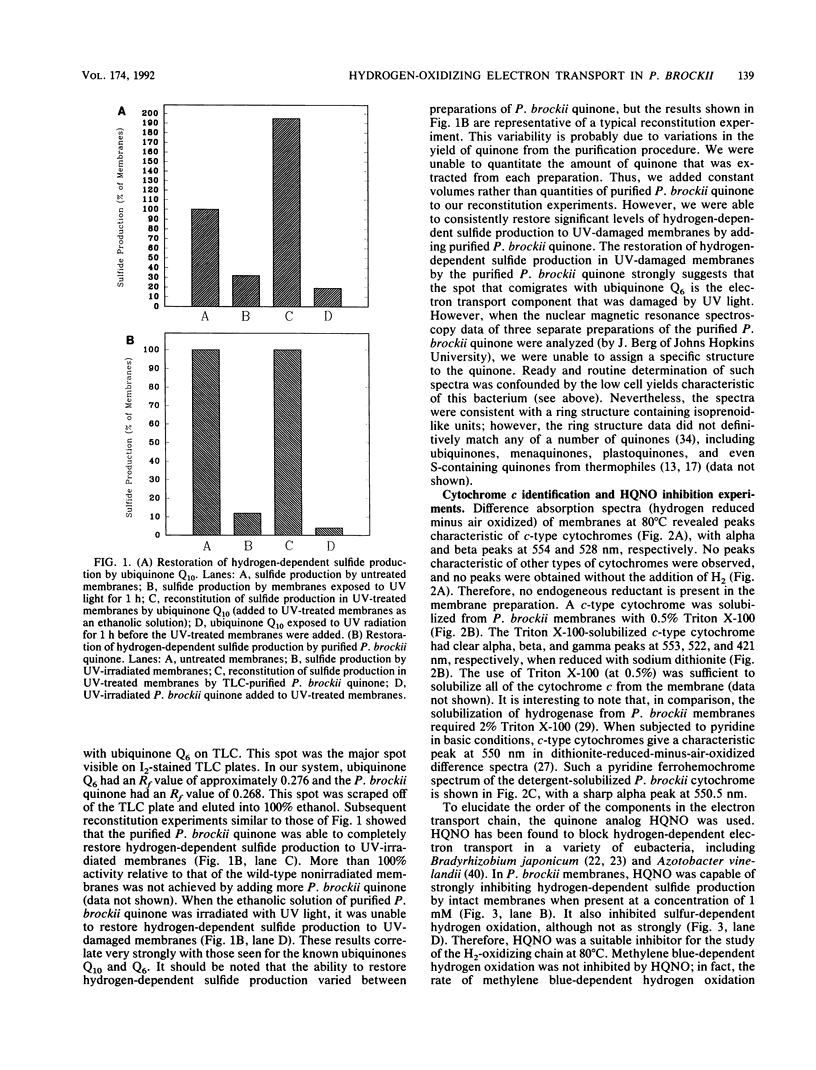
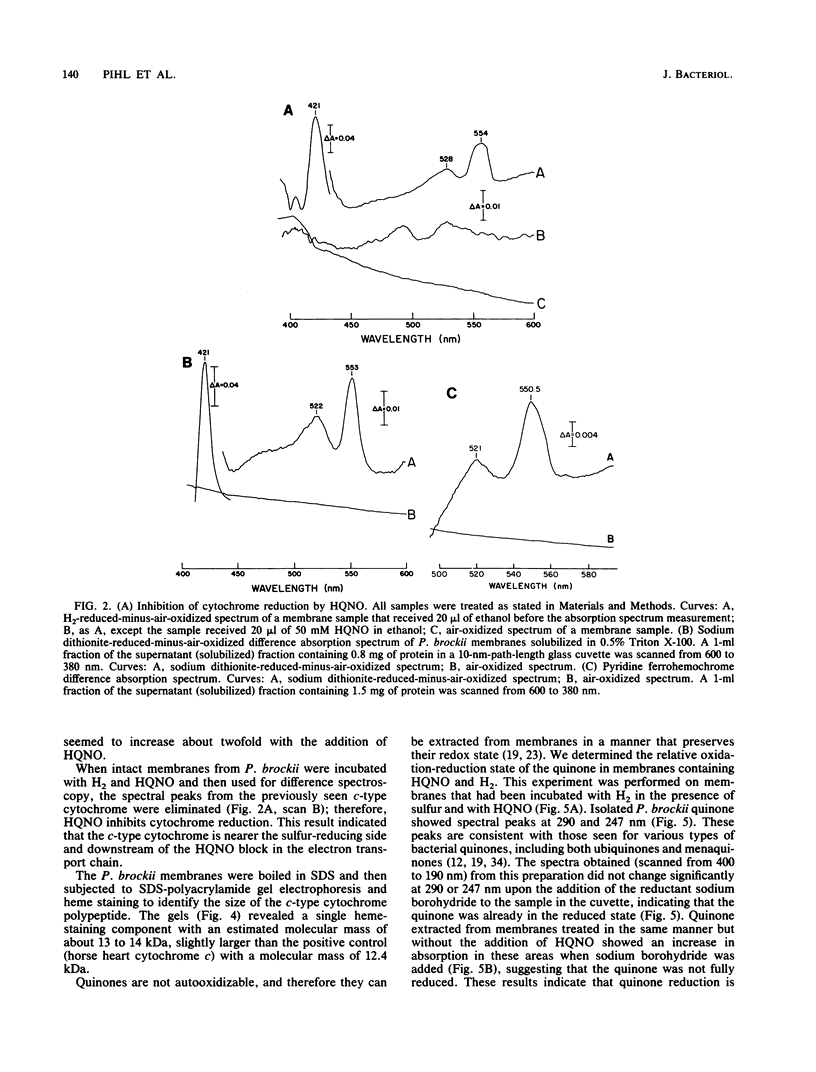
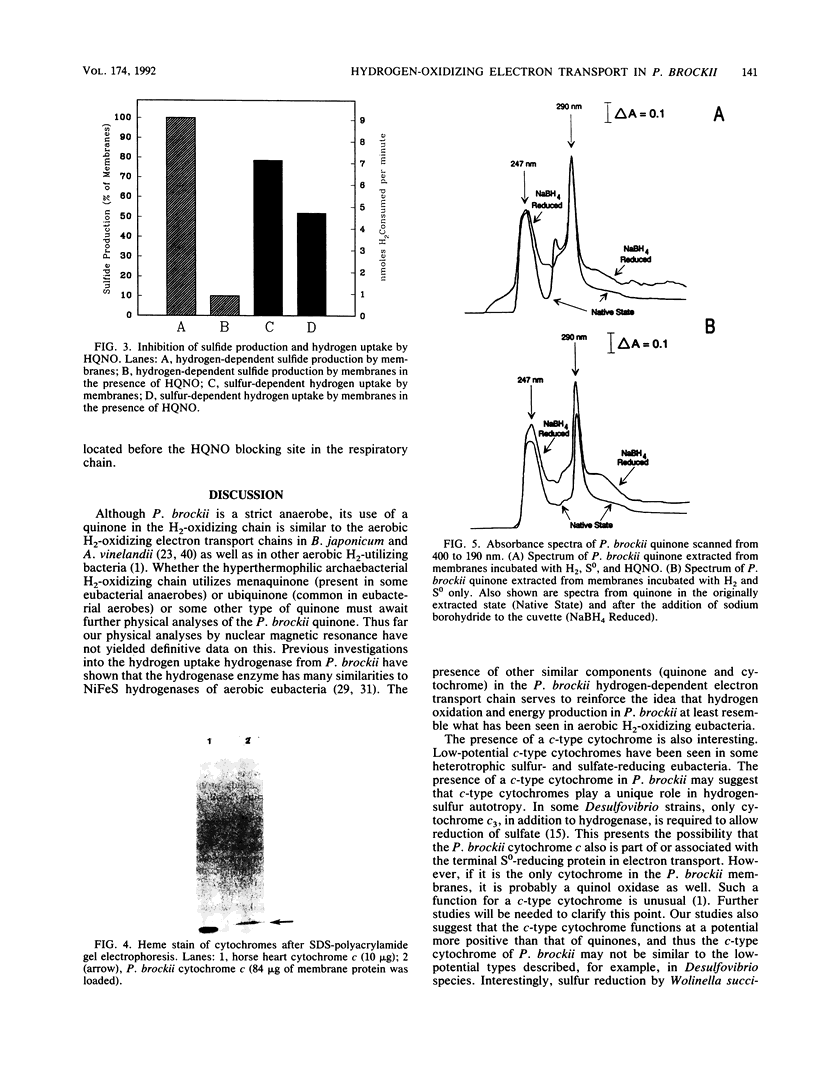
The hyperthermophilic archaebacterium Pyrodictium brockii grows optimally at 105 degrees C by a form of metabolism known as hydrogen-sulfur autotrophy, which is characterized by the oxidation of H2 by S0 to produce ATP and H2S. UV-irradiated membranes were not able to carry out the hydrogen-dependent reduction of sulfur. However, the activity could be restored by the addition of ubiquinone Q10 or ubiquinone Q6 to the UV-damaged membranes. A quinone with thin-layer chromatography migration properties similar to those of Q6 was purified by thin-layer chromatography from membranes of P. brockii, but nuclear magnetic resonance analysis failed to confirm its identity as a ubiquinone. P. brockii quinone was capable of restoring hydrogen-dependent sulfur reduction to UV-irradiated membranes. Hydrogen-reduced-minus-air-oxidized absorption difference spectra on membranes revealed absorption peaks characteristic of c-type cytochromes. A c-type cytochrome with alpha, beta, and gamma peaks at 553, 522, and 421 nm, respectively, was solubilized from membranes with 0.5% Triton X-100. Pyridine ferrohemochrome spectra confirmed its identity as a c-type cytochrome, and heme staining of membranes loaded on sodium dodecyl sulfate gels revealed a single heme-containing component of 13 to 14 kDa. Studies with the ubiquinone analog 2-n-heptyl-4-hydroxyquinoline-N-oxide demonstrated that the P. brockii quinone is located on the substrate side of the electron transport chain with respect to the c-type cytochrome. These first characterizations of the strictly anaerobic, presumably primitive P. brockii electron transport chain suggest that the hydrogenase operates at a relatively high redox potential and that the H2-oxidizing chain more closely resembles those of aerobic eubacterial H2-oxidizing bacteria than those of the H2-metabolizing systems of anaerobes or the hyperthermophile Pyrococcus furiosus.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anraku Y. Bacterial electron transport chains. Annu Rev Biochem. 1988;57:101–132. doi: 10.1146/annurev.bi.57.070188.000533. [DOI] [PubMed] [Google Scholar]
- Aono S., Bryant F. O., Adams M. W. A novel and remarkably thermostable ferredoxin from the hyperthermophilic archaebacterium Pyrococcus furiosus. J Bacteriol. 1989 Jun;171(6):3433–3439. doi: 10.1128/jb.171.6.3433-3439.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Blumentals I. I., Itoh M., Olson G. J., Kelly R. M. Role of Polysulfides in Reduction of Elemental Sulfur by the Hyperthermophilic Archaebacterium Pyrococcus furiosus. Appl Environ Microbiol. 1990 May;56(5):1255–1262. doi: 10.1128/aem.56.5.1255-1262.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumentals I. I., Robinson A. S., Kelly R. M. Characterization of sodium dodecyl sulfate-resistant proteolytic activity in the hyperthermophilic archaebacterium Pyrococcus furiosus. Appl Environ Microbiol. 1990 Jul;56(7):1992–1998. doi: 10.1128/aem.56.7.1992-1998.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown S. H., Costantino H. R., Kelly R. M. Characterization of Amylolytic Enzyme Activities Associated with the Hyperthermophilic Archaebacterium Pyrococcus furiosus. Appl Environ Microbiol. 1990 Jul;56(7):1985–1991. doi: 10.1128/aem.56.7.1985-1991.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant F. O., Adams M. W. Characterization of hydrogenase from the hyperthermophilic archaebacterium, Pyrococcus furiosus. J Biol Chem. 1989 Mar 25;264(9):5070–5079. [PubMed] [Google Scholar]
- Conover R. C., Kowal A. T., Fu W. G., Park J. B., Aono S., Adams M. W., Johnson M. K. Spectroscopic characterization of the novel iron-sulfur cluster in Pyrococcus furiosus ferredoxin. J Biol Chem. 1990 May 25;265(15):8533–8541. [PubMed] [Google Scholar]
- Costantino H. R., Brown S. H., Kelly R. M. Purification and characterization of an alpha-glucosidase from a hyperthermophilic archaebacterium, Pyrococcus furiosus, exhibiting a temperature optimum of 105 to 115 degrees C. J Bacteriol. 1990 Jul;172(7):3654–3660. doi: 10.1128/jb.172.7.3654-3660.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Rosa M., De Rosa S., Gambacorta A., Minale L. Caldariellaquinone, a unique benzo(b)thiophen-4,7-quinone from Caldariella acidophila, an extremely thermophilic and acidophilic bacterium. J Chem Soc Perkin 1. 1977;(6):653–657. doi: 10.1039/p19770000653. [DOI] [PubMed] [Google Scholar]
- Erickson S. K., Parker G. L. The electron-transport system of Micrococcus lutea (Sarcina lutea). Biochim Biophys Acta. 1969 May;180(1):56–62. doi: 10.1016/0005-2728(69)90193-5. [DOI] [PubMed] [Google Scholar]
- Fauque G., Herve D., Le Gall J. Structure-function relationship in hemoproteins: the role of cytochrome c3 in the reduction of colloidal sulfur by sulfate-reducing bacteria. Arch Microbiol. 1979 Jun;121(3):261–264. doi: 10.1007/BF00425065. [DOI] [PubMed] [Google Scholar]
- Francis R. T., Jr, Becker R. R. Specific indication of hemoproteins in polyacrylamide gels using a double-staining process. Anal Biochem. 1984 Feb;136(2):509–514. doi: 10.1016/0003-2697(84)90253-7. [DOI] [PubMed] [Google Scholar]
- Ishii M., Kawasumi T., Igarashi Y., Kodama T., Minoda Y. 2-Methylthio-1,4-naphthoquinone, a unique sulfur-containing quinone from a thermophilic hydrogen-oxidizing bacterium, Hydrogenobacter thermophilus. J Bacteriol. 1987 Jun;169(6):2380–2384. doi: 10.1128/jb.169.6.2380-2384.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kröger A. Determination of contents and redox states of ubiquinone and menaquinone. Methods Enzymol. 1978;53:579–591. doi: 10.1016/s0076-6879(78)53059-0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Maier R. J., Merberg D. M. Rhizobium japonicum mutants that are hypersensitive to repression of H2 uptake by oxygen. J Bacteriol. 1982 Apr;150(1):161–167. doi: 10.1128/jb.150.1.161-167.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brian M. R., Maier R. J. Electron transport components involved in hydrogen oxidation in free-living Rhizobium japonicum. J Bacteriol. 1982 Oct;152(1):422–430. doi: 10.1128/jb.152.1.422-430.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brian M. R., Maier R. J. Role of ubiquinone in hydrogen-dependent electron transport in Rhizobium japonicum. J Bacteriol. 1985 Feb;161(2):775–777. doi: 10.1128/jb.161.2.775-777.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odom J. M., Peck H. D., Jr Hydrogenase, electron-transfer proteins, and energy coupling in the sulfate-reducing bacteria Desulfovibrio. Annu Rev Microbiol. 1984;38:551–592. doi: 10.1146/annurev.mi.38.100184.003003. [DOI] [PubMed] [Google Scholar]
- Parameswaran A. K., Provan C. N., Sturm F. J., Kelly R. M. Sulfur Reduction by the Extremely Thermophilic Archaebacterium Pyrodictium occultum. Appl Environ Microbiol. 1987 Jul;53(7):1690–1693. doi: 10.1128/aem.53.7.1690-1693.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phipps B. M., Hoffmann A., Stetter K. O., Baumeister W. A novel ATPase complex selectively accumulated upon heat shock is a major cellular component of thermophilic archaebacteria. EMBO J. 1991 Jul;10(7):1711–1722. doi: 10.1002/j.1460-2075.1991.tb07695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pihl T. D., Maier R. J. Purification and characterization of the hydrogen uptake hydrogenase from the hyperthermophilic archaebacterium Pyrodictium brockii. J Bacteriol. 1991 Mar;173(6):1839–1844. doi: 10.1128/jb.173.6.1839-1844.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pihl T. D., Schicho R. N., Black L. K., Schulman B. A., Maier R. J., Kelly R. M. Hydrogen-sulfur autotrophy in the hyperthermophilic archaebacterium, Pyrodictium brockii. Biotechnol Genet Eng Rev. 1990;8:345–377. doi: 10.1080/02648725.1990.10647874. [DOI] [PubMed] [Google Scholar]
- Pihl T. D., Schicho R. N., Kelly R. M., Maier R. J. Characterization of hydrogen-uptake activity in the hyperthermophile Pyrodictium brockii. Proc Natl Acad Sci U S A. 1989 Jan;86(1):138–141. doi: 10.1073/pnas.86.1.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer P., Kofler M. Physicochemical properties and methods of analysis of phylloquinones, menaquinones, ubiquinones, plastoquinones, menadione, and related compounds. Vitam Horm. 1966;24:349–399. doi: 10.1016/s0083-6729(08)60211-3. [DOI] [PubMed] [Google Scholar]
- Stults L. W., O'Hara E. B., Maier R. J. Nickel is a component of hydrogenase in Rhizobium japonicum. J Bacteriol. 1984 Jul;159(1):153–158. doi: 10.1128/jb.159.1.153-158.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thauer R. K., Jungermann K., Decker K. Energy conservation in chemotrophic anaerobic bacteria. Bacteriol Rev. 1977 Mar;41(1):100–180. doi: 10.1128/br.41.1.100-180.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong T. Y., Maier R. J. Hydrogen-oxidizing electron transport components in nitrogen-fixing Azotobacter vinelandii. J Bacteriol. 1984 Jul;159(1):348–352. doi: 10.1128/jb.159.1.348-352.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]




